Sex- and brain region-specific acceleration of β-amyloidogenesis following behavioral stress in a mouse model of Alzheimer's disease
- PMID: 21059265
- PMCID: PMC2988063
- DOI: 10.1186/1756-6606-3-34
Sex- and brain region-specific acceleration of β-amyloidogenesis following behavioral stress in a mouse model of Alzheimer's disease
Abstract
Background: It is hypothesized that complex interactions between multiple environmental factors and genetic factors are implicated in sporadic Alzheimer's disease (AD); however, the underlying mechanisms are poorly understood. Importantly, recent evidence reveals that expression and activity levels of the β-site APP cleaving enzyme 1 (BACE1), which initiates amyloid-β (Aβ) production, are elevated in AD brains. In this study, we investigated a molecular mechanism by which sex and stress interactions may accelerate β-amyloidogenesis and contribute to sporadic AD.
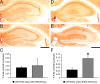
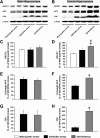
Results: We applied 5-day restraint stress (6 h/day) to the male and female 5XFAD transgenic mouse model of AD at the pre-pathological stage of disease, which showed little amyloid deposition under non-stressed control conditions. Exposure to the relatively brief behavioral stress increased levels of neurotoxic Aβ42 peptides, the β-secretase-cleaved C-terminal fragment (C99) and plaque burden in the hippocampus of female 5XFAD mice but not in that of male 5XFAD mice. In contrast, significant changes in the parameters of β-amyloidosis were not observed in the cerebral cortex of stressed male or female 5XFAD mice. We found that this sex- and brain region-specific acceleration of β-amyloidosis was accounted for by elevations in BACE1 and APP levels in response to adverse stress. Furthermore, not only BACE1 mRNA but also phosphorylation of the translation initiation factor eIF2α (a proposed mediator of the post-transcriptional upregulation of BACE1) was elevated in the hippocampus of stressed female 5XFAD mice.
Conclusions: Our results suggest that the higher prevalence of sporadic AD in women may be attributable to the vulnerability of female brains (especially, the hippocampus) to stressful events, which alter APP processing to favor the β-amyloidogenesis through the transcriptional and translational upregulation of BACE1 combined with elevations in its substrate APP.
Figures



Similar articles
-
Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer's disease.PLoS One. 2012;7(3):e32792. doi: 10.1371/journal.pone.0032792. Epub 2012 Mar 5. PLoS One. 2012. PMID: 22403710 Free PMC article.
-
Genetic inhibition of phosphorylation of the translation initiation factor eIF2α does not block Aβ-dependent elevation of BACE1 and APP levels or reduce amyloid pathology in a mouse model of Alzheimer's disease.PLoS One. 2014 Jul 3;9(7):e101643. doi: 10.1371/journal.pone.0101643. eCollection 2014. PLoS One. 2014. PMID: 24992504 Free PMC article.
-
Effects of BACE1 haploinsufficiency on APP processing and Aβ concentrations in male and female 5XFAD Alzheimer mice at different disease stages.Neuroscience. 2015 Oct 29;307:128-37. doi: 10.1016/j.neuroscience.2015.08.037. Epub 2015 Aug 24. Neuroscience. 2015. PMID: 26314636 Free PMC article.
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
Cited by
-
Sex and Gender Differences in Alzheimer's Disease: Genetic, Hormonal, and Inflammation Impacts.Int J Mol Sci. 2024 Aug 3;25(15):8485. doi: 10.3390/ijms25158485. Int J Mol Sci. 2024. PMID: 39126053 Free PMC article. Review.
-
7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer's disease.Neuropsychopharmacology. 2012 Jan;37(2):434-44. doi: 10.1038/npp.2011.191. Epub 2011 Sep 7. Neuropsychopharmacology. 2012. PMID: 21900882 Free PMC article.
-
Dysregulated ac4C modification of mRNA in a mouse model of early-stage Alzheimer's disease.Cell Biosci. 2025 Apr 13;15(1):45. doi: 10.1186/s13578-025-01389-8. Cell Biosci. 2025. PMID: 40223095 Free PMC article.
-
The Impact of High-Dose Fish Oil Supplementation on Mfsd2a, Aqp4, and Amyloid-β Expression in Retinal Blood Vessels of 5xFAD Alzheimer's Mouse Model.Int J Mol Sci. 2024 Aug 29;25(17):9400. doi: 10.3390/ijms25179400. Int J Mol Sci. 2024. PMID: 39273347 Free PMC article.
-
A small molecule transcription factor EB activator ameliorates beta-amyloid precursor protein and Tau pathology in Alzheimer's disease models.Aging Cell. 2020 Feb;19(2):e13069. doi: 10.1111/acel.13069. Epub 2019 Dec 19. Aging Cell. 2020. PMID: 31858697 Free PMC article.
References
-
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

