A randomized phase III study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974)
- PMID: 21059637
- PMCID: PMC3082158
- DOI: 10.1093/annonc/mdq575
A randomized phase III study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974)
Abstract
Background: To compare the efficacy of one cycle of standard dose cisplatin, etoposide, and ifosfamide (VIP) plus three cycles of high-dose VIP followed by stem-cell infusion [high-dose chemotherapy (HD-CT arm)] to four cycles of standard cisplatin, etoposide, and bleomycin (BEP) in patients with poor-prognosis germ-cell cancer (GCC).
Patient and methods: Patients with poor-prognosis GCC were assigned to receive either BEP or VIP followed by HD-CT. To show a 15% improvement in a 1-year failure-free survival (FFS), the study aimed to recruit 222 patients but closed with 137, due to slow accrual.
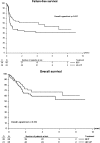
Results: One hundred thirty-one patients were included in this analysis. The complete response rates in the HD-CT and in the BEP arm did not differ: (intention to treat) 44.6% versus 33.3% (P = 0.18). There was no difference in FFS between the two treatment arms (P = 0.057, 66 events). At 2 years, the FFS rate was 44.8% [95% confidence interval (CI) 32.5-56.4] and 58.2%, respectively (95% CI 48.0-71.9); but this 16.3% (standard deviation 7.5%) difference was not statistically significant (P = 0.060). Overall survival did not differ between the two groups (log-rank P > 0.1, 47 deaths).
Conclusion: This study could not demonstrate that high-dose chemotherapy given as part of first-line therapy improves outcome in patients with poor-prognosis GCC.
Figures
References
-
- International Germ Cell Collaborative Group. International Germ Cell Classification: a prognostic factor-based staging system for metastatic germ-cell cancers. J Clin Oncol. 1997;15:594–603. - PubMed
-
- Bokemeyer C, Schmoll HJ, Illinger HJ, et al. A phase I/II study of stepwise dose-escalet regimen of cisplatin, etoposide and ifosfamide plus granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with advanced germ cell tumours. Eur J Cancer. 1993;29:2225–2231. - PubMed
-
- Schmoll HJ, Kollmannsberger C, Metzner B, et al. Long-term results of first-line sequential high-dose etoposide, ifosfamide, and cisplatin chemotherapy plus autologous stem cell support for patients with advanced metastatic germ cell cancer: an extended phase I/II study of the German Testicular Cancer Study Group. J Clin Oncol. 2003;21:4083–4091. - PubMed
-
- Bokemeyer C, Kollmannsberger C, Meisner C, et al. First-line high-dose chemotherapy compared with standard-dose PEB/VIP chemotherapy in patients with advanced germ cell tumors: a multivariate and matched-pair analysis. J Clin Oncol. 1999;17:3450–3456. - PubMed
-
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 5U10 CA11488-32/CA/NCI NIH HHS/United States
- 5U10 CA11488-27/CA/NCI NIH HHS/United States
- 5U10 CA11488-38/CA/NCI NIH HHS/United States
- 5U10 CA11488-39/CA/NCI NIH HHS/United States
- 5U10 CA11488-28/CA/NCI NIH HHS/United States
- 5U10 CA11488-35/CA/NCI NIH HHS/United States
- 5U10 CA11488-31/CA/NCI NIH HHS/United States
- 5U10 CA11488-37/CA/NCI NIH HHS/United States
- 5U10 CA11488-29/CA/NCI NIH HHS/United States
- 5U10 CA11488-40/CA/NCI NIH HHS/United States
- 5U10 CA11488-36/CA/NCI NIH HHS/United States
- 5U10 CA11488-33/CA/NCI NIH HHS/United States
- 5U10 CA11488-34/CA/NCI NIH HHS/United States
- 5U10 CA11488-30/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical