Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II
- PMID: 21059645
- PMCID: PMC3030374
- DOI: 10.1074/jbc.M110.186643
Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II
Abstract
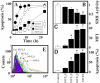
Mitochondrial complex II (CII) has been recently identified as a novel target for anti-cancer drugs. Mitochondrially targeted vitamin E succinate (MitoVES) is modified so that it is preferentially localized to mitochondria, greatly enhancing its pro-apoptotic and anti-cancer activity. Using genetically manipulated cells, MitoVES caused apoptosis and generation of reactive oxygen species (ROS) in CII-proficient malignant cells but not their CII-dysfunctional counterparts. MitoVES inhibited the succinate dehydrogenase (SDH) activity of CII with IC(50) of 80 μM, whereas the electron transfer from CII to CIII was inhibited with IC(50) of 1.5 μM. The agent had no effect either on the enzymatic activity of CI or on electron transfer from CI to CIII. Over 24 h, MitoVES caused stabilization of the oxygen-dependent destruction domain of HIF1α fused to GFP, indicating promotion of the state of pseudohypoxia. Molecular modeling predicted the succinyl group anchored into the proximal CII ubiquinone (UbQ)-binding site and successively reduced interaction energies for serially shorter phytyl chain homologs of MitoVES correlated with their lower effects on apoptosis induction, ROS generation, and SDH activity. Mutation of the UbQ-binding Ser(68) within the proximal site of the CII SDHC subunit (S68A or S68L) suppressed both ROS generation and apoptosis induction by MitoVES. In vivo studies indicated that MitoVES also acts by causing pseudohypoxia in the context of tumor suppression. We propose that mitochondrial targeting of VES with an 11-carbon chain localizes the agent into an ideal position across the interface of the mitochondrial inner membrane and matrix, optimizing its biological effects as an anti-cancer drug.
Figures





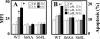


Similar articles
-
Reactive oxygen species are generated by the respiratory complex II--evidence for lack of contribution of the reverse electron flow in complex I.FEBS J. 2013 Feb;280(3):927-38. doi: 10.1111/febs.12086. Epub 2013 Jan 7. FEBS J. 2013. PMID: 23206332
-
Ubiquinone-binding site mutagenesis reveals the role of mitochondrial complex II in cell death initiation.Cell Death Dis. 2015 May 7;6(5):e1749. doi: 10.1038/cddis.2015.110. Cell Death Dis. 2015. PMID: 25950479 Free PMC article.
-
Alpha-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II.Oncogene. 2008 Jul 17;27(31):4324-35. doi: 10.1038/onc.2008.69. Epub 2008 Mar 31. Oncogene. 2008. PMID: 18372923 Free PMC article.
-
Mitocans as anti-cancer agents targeting mitochondria: lessons from studies with vitamin E analogues, inhibitors of complex II.J Bioenerg Biomembr. 2007 Feb;39(1):65-72. doi: 10.1007/s10863-006-9060-z. J Bioenerg Biomembr. 2007. PMID: 17294131 Review.
-
Mitochondrial complex II and reactive oxygen species in disease and therapy.Redox Rep. 2020 Dec;25(1):26-32. doi: 10.1080/13510002.2020.1752002. Redox Rep. 2020. PMID: 32290794 Free PMC article. Review.
Cited by
-
Combining Photodynamic Therapy and Targeted Drug Delivery Systems: Enhancing Mitochondrial Toxicity for Improved Cancer Outcomes.Int J Mol Sci. 2024 Oct 8;25(19):10796. doi: 10.3390/ijms251910796. Int J Mol Sci. 2024. PMID: 39409125 Free PMC article. Review.
-
Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death.Cancer Res. 2012 May 15;72(10):2634-44. doi: 10.1158/0008-5472.CAN-11-3928. Epub 2012 Mar 19. Cancer Res. 2012. PMID: 22431711 Free PMC article.
-
Oncostatic-Cytoprotective Effect of Melatonin and Other Bioactive Molecules: A Common Target in Mitochondrial Respiration.Int J Mol Sci. 2016 Mar 7;17(3):341. doi: 10.3390/ijms17030341. Int J Mol Sci. 2016. PMID: 26959015 Free PMC article. Review.
-
Multifunctional decoration of alpha-tocopheryl succinate-based NP for cancer treatment: effect of TPP and LTVSPWY peptide.J Mater Sci Mater Med. 2017 Aug 31;28(10):152. doi: 10.1007/s10856-017-5963-y. J Mater Sci Mater Med. 2017. PMID: 28861765
-
Hierarchical pulmonary target nanoparticles via inhaled administration for anticancer drug delivery.Drug Deliv. 2017 Nov;24(1):1191-1203. doi: 10.1080/10717544.2017.1365395. Drug Deliv. 2017. PMID: 28844172 Free PMC article.
References
-
- Fantin V. R., Leder P. (2006) Oncogene 25, 4787–4797 - PubMed
-
- Moreno-Sánchez R., Rodríguez-Enríquez S., Marín-Hernández A., Saavedra E. (2007) FEBS J. 274, 1393–1418 - PubMed
-
- Gogvadze V., Orrenius S., Zhivotovsky B. (2008) Trends Cell Biol. 18, 165–173 - PubMed
-
- Trachootham D., Alexandre J., Huang P. (2009) Nat. Rev. Drug Discov. 8, 579–591 - PubMed
-
- Fulda S., Galluzzi L., Kroemer G. (2010) Nat. Rev. Drug Discov. 9, 447–464 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

