MicroRNA-19 (miR-19) regulates tissue factor expression in breast cancer cells
- PMID: 21059650
- PMCID: PMC3020751
- DOI: 10.1074/jbc.M110.146530
MicroRNA-19 (miR-19) regulates tissue factor expression in breast cancer cells
Abstract
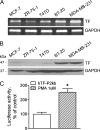
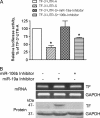
Tissue factor has been recognized as a regulator of tumor angiogenesis and metastasis. The tissue factor gene is selectively expressed in highly invasive breast cancer cells, and the mechanisms regulating tissue factor expression in these cells remain unclear. This study demonstrates that microRNA-19 (miR-19) regulates tissue factor expression in breast cancer cells, providing a molecular basis for the selective expression of the tissue factor gene. Tissue factor protein was barely detectable in MCF-7, T47D, and ZR-75-1 cells (less invasive breast lines) but was expressed at a significantly higher level in MDA-MB-231 and BT-20 cells (invasive breast lines) as assayed by Western blot. The tissue factor gene promoter was activated, and forced expression of tissue factor cDNA was achieved in MCF-7 cells, implying that the 3'-UTR of the tissue factor transcript is responsible for the suppression of tissue factor expression. Bioinformatics analysis predicted microRNA-binding sites for miR-19, miR-20, and miR-106b in the 3'-UTR of the tissue factor transcript. Reporter gene assay using the TF-3'-UTR luciferase reporter construct confirmed that the 3'-UTR negatively regulates gene expression in MCF-7 cells, an effect reversed by deletion of the miR-19-binding site. Application of the miR-19 inhibitor induces endogenous tissue factor expression in MCF-7 cells, and overexpression of miR-19 down-regulates tissue factor expression in MDA-MB-231 cells. RT-PCR analysis using cDNA made from Ago2-immunoprecipitated RNA samples confirmed that Ago2 binds preferentially to tissue factor 3'-UTR in MCF-7 cells, as compared with MDA-MB-231 cells, consistent with the observation that miR-19 levels are higher in MCF-7 cells.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

