Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice
- PMID: 21059750
- PMCID: PMC3023387
- DOI: 10.1096/fj.10-171488
Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice
Abstract
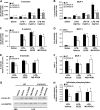
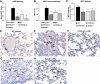
Cytochrome P-450 (CYP)-derived epoxyeicosatrienoic acids (EETs) possess potent anti-inflammatory effects in vitro. However, the effect of increased CYP-mediated EET biosynthesis and decreased soluble epoxide hydrolase (sEH, Ephx2)-mediated EET hydrolysis on vascular inflammation in vivo has not been rigorously investigated. Consequently, we characterized acute vascular inflammatory responses to endotoxin in transgenic mice with endothelial expression of the human CYP2J2 and CYP2C8 epoxygenases and mice with targeted disruption of Ephx2. Compared to wild-type controls, CYP2J2 transgenic, CYP2C8 transgenic, and Ephx2(-/-) mice each exhibited a significant attenuation of endotoxin-induced activation of nuclear factor (NF)-κB signaling, cellular adhesion molecule, chemokine and cytokine expression, and neutrophil infiltration in lung in vivo. Furthermore, attenuation of endotoxin-induced NF-κB activation and cellular adhesion molecule and chemokine expression was observed in primary pulmonary endothelial cells isolated from CYP2J2 and CYP2C8 transgenic mice. This attenuation was inhibited by a putative EET receptor antagonist and CYP epoxygenase inhibitor, directly implicating CYP epoxygenase-derived EETs with the observed anti-inflammatory phenotype. Collectively, these data demonstrate that potentiation of the CYP epoxygenase pathway by either increased endothelial EET biosynthesis or globally decreased EET hydrolysis attenuates NF-κB-dependent vascular inflammatory responses in vivo and may serve as a viable anti-inflammatory therapeutic strategy.
Figures



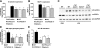
References
-
- Lawrence T., Willoughby D. A., Gilroy D. W. (2002) Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2, 787–795 - PubMed
-
- Ghosh S., May M. J., Kopp E. B. (1998) NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 - PubMed
-
- Carlsen H., Moskaug J. O., Fromm S. H., Blomhoff R. (2002) In vivo imaging of NF-κB activity. J. Immunol. 168, 1441–1446 - PubMed
-
- Barnes P. J., Karin M. (1997) Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336, 1066–1071 - PubMed
-
- Liu S. F., Malik A. B. (2006) NF-κB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L622–L645 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

