Integration and propagation of somatosensory responses in the corticostriatal pathway: an intracellular study in vivo
- PMID: 21059765
- PMCID: PMC3043532
- DOI: 10.1113/jphysiol.2010.199646
Integration and propagation of somatosensory responses in the corticostriatal pathway: an intracellular study in vivo
Abstract
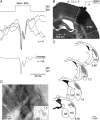
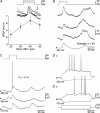
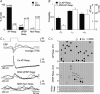
The dorsolateral striatum is critically involved in the execution and learning of sensorimotor tasks. It is proposed that this striatal function is achieved by the integration of convergent somatosensory and motor corticostriatal (CS) inputs in striatal medium-spiny neurons (MSNs). However, the cellular mechanisms of integration and propagation of somatosensory information in the CS pathway remain unknown. Here, by means of in vivo intracellular recordings in the rat, we analysed how sensory events generated by multi-whisker deflection, which provide essential somaesthetic information in rodents, are processed in contralateral barrel cortex layer 5 neurons and in the related somatosensory striatal MSNs. Pyramidal layer 5 barrel cortex neurons, including neurons antidromically identified as CS, responded to whisker deflection by depolarizing post-synaptic potentials that could reliably generate action potential discharge. In contrast, only half of recorded somatosensory striatal MSNs displayed whisker-evoked synaptic depolarizations that were effective in eliciting action potentials in one-third of responding neurons. The remaining population of MSNs did not exhibit any detectable electrical events in response to whisker stimulation. The relative inconstancy of sensory-evoked responses in MSNs was due, at least in part, to a Cl(-)-dependent membrane conductance concomitant with the cortical inputs,which was probably caused by whisker-induced activation of striatal GABAergic interneurons. Our results suggest that the propagation of whisker-mediated sensory flow through the CS pathway results in a refinement of sensory information in the striatum, which might allow the selection of specific sets of MSNs that are functionally significant during a given somaesthetic-guided behaviour.
Figures





Similar articles
-
Converging sensory and motor cortical inputs onto the same striatal neurons: An in vivo intracellular investigation.PLoS One. 2020 Feb 5;15(2):e0228260. doi: 10.1371/journal.pone.0228260. eCollection 2020. PLoS One. 2020. PMID: 32023274 Free PMC article.
-
Repeated whisker stimulation evokes invariant neuronal responses in the dorsolateral striatum of anesthetized rats: a potential correlate of sensorimotor habits.J Neurophysiol. 2011 May;105(5):2225-38. doi: 10.1152/jn.01018.2010. Epub 2011 Mar 9. J Neurophysiol. 2011. PMID: 21389309 Free PMC article.
-
Identification of the source of the bilateral projection system from cortex to somatosensory neostriatum and an exploration of its physiological actions.Neuroscience. 2001;103(1):87-96. doi: 10.1016/s0306-4522(00)00564-9. Neuroscience. 2001. PMID: 11311789
-
From single cells and single columns to cortical networks: dendritic excitability, coincidence detection and synaptic transmission in brain slices and brains.Exp Physiol. 2017 May 1;102(5):489-521. doi: 10.1113/EP085776. Epub 2017 Apr 21. Exp Physiol. 2017. PMID: 28139019 Free PMC article. Review.
-
Intracortical processes regulating the integration of sensory information.Prog Brain Res. 1990;86:129-41. doi: 10.1016/s0079-6123(08)63172-6. Prog Brain Res. 1990. PMID: 1982365 Review.
Cited by
-
Populations of striatal medium spiny neurons encode vibrotactile frequency in rats: modulation by slow wave oscillations.J Neurophysiol. 2013 Jan;109(2):315-20. doi: 10.1152/jn.00489.2012. Epub 2012 Oct 31. J Neurophysiol. 2013. PMID: 23114217 Free PMC article.
-
Cellular and Synaptic Dysfunctions in Parkinson's Disease: Stepping out of the Striatum.Cells. 2019 Aug 29;8(9):1005. doi: 10.3390/cells8091005. Cells. 2019. PMID: 31470672 Free PMC article. Review.
-
Selective Increase of Auditory Cortico-Striatal Coherence during Auditory-Cued Go/NoGo Discrimination Learning.Front Behav Neurosci. 2016 Jan 11;9:368. doi: 10.3389/fnbeh.2015.00368. eCollection 2015. Front Behav Neurosci. 2016. PMID: 26793085 Free PMC article.
-
Migraine attacks the Basal Ganglia.Mol Pain. 2011 Sep 21;7:71. doi: 10.1186/1744-8069-7-71. Mol Pain. 2011. PMID: 21936901 Free PMC article.
-
Distinct Corticostriatal and Intracortical Pathways Mediate Bilateral Sensory Responses in the Striatum.Cereb Cortex. 2016 Dec;26(12):4405-4415. doi: 10.1093/cercor/bhw268. Epub 2016 Sep 24. Cereb Cortex. 2016. PMID: 27664965 Free PMC article.
References
-
- Alloway KD, Lou L, Nwabueze-Ogbo F, Chakrabarti S. Topography of cortical projections to the dorsolateral neostriatum in rats: multiple overlapping sensorimotor pathways. J Comp Neurol. 2006;499:33–48. - PubMed
-
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. - PubMed
-
- Brecht M. Barrel cortex and whisker-mediated behaviors. Curr Opin Neurobiol. 2007;17:408–416. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

