Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome
- PMID: 21059844
- PMCID: PMC2983070
- DOI: 10.1083/jcb.201007107
Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome
Abstract
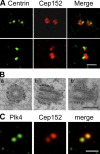
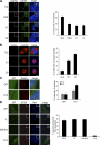
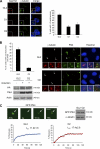
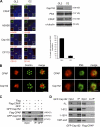
Both gain and loss of function studies have identified the Polo-like kinase Plk4/Sak as a crucial regulator of centriole biogenesis, but the mechanisms governing centrosome duplication are incompletely understood. In this study, we show that the pericentriolar material protein, Cep152, interacts with the distinctive cryptic Polo-box of Plk4 via its N-terminal domain and is required for Plk4-induced centriole overduplication. Reduction of endogenous Cep152 levels results in a failure in centriole duplication, loss of centrioles, and formation of monopolar mitotic spindles. Interfering with Cep152 function prevents recruitment of Plk4 to the centrosome and promotes loss of CPAP, a protein required for the control of centriole length in Plk4-regulated centriole biogenesis. Our results suggest that Cep152 recruits Plk4 and CPAP to the centrosome to ensure a faithful centrosome duplication process.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

