Distinctive functions of p160 steroid receptor coactivators in proliferation of an estrogen-independent, tamoxifen-resistant breast cancer cell line
- PMID: 21059860
- PMCID: PMC3014261
- DOI: 10.1677/ERC-09-0285
Distinctive functions of p160 steroid receptor coactivators in proliferation of an estrogen-independent, tamoxifen-resistant breast cancer cell line
Abstract
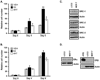
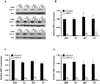
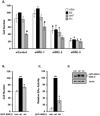
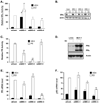
Elevated expression of steroid receptor coactivator-3 (SRC-3), a member of the p160 family of nuclear receptor coactivators, has been implicated in tamoxifen resistance of breast tumors while the involvement of the two other members of this family, SRC-1 and SRC-2, is less well characterized. In this study, using small interfering RNA-based silencing, the role of each SRC coactivator in the growth of the LCC2 estrogen-independent and tamoxifen-resistant breast cancer cell line was evaluated. The loss of SRC-1, SRC-2, or SRC-3 did not significantly alter LCC2 proliferation or cell cycle distribution of 4-hydroxytamoxifen- versus vehicle-treated cells. However, depletion of SRC-2 and SRC-3, but not SRC-1, decreased basal cell proliferation and increased apoptosis. Cell cycle analyses further illustrated the divergent contributions of SRC-2 and SRC-3 with depletion of the former increasing the percentage of cells in the G(0)G(1) and sub-G(0)G(1) phases of cell cycle yet maintaining sensitivity to estradiol and ICI 182 780 antiestrogen, while SRC-3 depletion increased cells in the sub-G(0)G(1) phase and ablated response to estrogen receptor α (ERα) ligands. Surprisingly, the effects of SRC coactivator depletion on ERα transcriptional activity, as measured by luciferase reporter gene, did not correspond to the observed effects on proliferation (e.g. SRC-1 knockdown increases ERα activity). Collectively, these data indicate that SRC control of basal and hormone-regulated proliferations is not solely mediated by ERα, and suggest that targeting growth inhibition by disrupting SRC-2 and SRC-3 function may be an effective approach to inhibit the growth of tamoxifen-resistant breast cancer.
Conflict of interest statement
Figures


Similar articles
-
Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-alpha-dependent and estrogen receptor-alpha-independent mechanisms.Mol Cancer Ther. 2008 Jul;7(7):2096-108. doi: 10.1158/1535-7163.MCT-07-2350. Mol Cancer Ther. 2008. PMID: 18645020 Free PMC article.
-
Unique roles of p160 coactivators for regulation of breast cancer cell proliferation and estrogen receptor-alpha transcriptional activity.Endocrinology. 2009 Apr;150(4):1588-96. doi: 10.1210/en.2008-1001. Epub 2008 Dec 18. Endocrinology. 2009. PMID: 19095746 Free PMC article.
-
Cooperative activation of cyclin D1 and progesterone receptor gene expression by the SRC-3 coactivator and SMRT corepressor.Mol Endocrinol. 2010 Jun;24(6):1187-202. doi: 10.1210/me.2009-0480. Epub 2010 Apr 14. Mol Endocrinol. 2010. PMID: 20392877 Free PMC article.
-
SRC-3 has a role in cancer other than as a nuclear receptor coactivator.Int J Biol Sci. 2011;7(5):664-72. doi: 10.7150/ijbs.7.664. Epub 2011 May 24. Int J Biol Sci. 2011. PMID: 21647249 Free PMC article. Review.
-
Thoughts on tamoxifen resistant breast cancer. Are coregulators the answer or just a red herring?J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):255-9. doi: 10.1016/s0960-0760(00)00101-1. J Steroid Biochem Mol Biol. 2000. PMID: 11162933 Review.
Cited by
-
Identification of key genes involved in tamoxifen-resistant breast cancer using bioinformatics analysis.Transl Cancer Res. 2021 Dec;10(12):5246-5257. doi: 10.21037/tcr-21-1276. Transl Cancer Res. 2021. PMID: 35116374 Free PMC article.
-
The role of AIB1 in breast cancer.Oncol Lett. 2012 Oct;4(4):588-594. doi: 10.3892/ol.2012.803. Epub 2012 Jul 16. Oncol Lett. 2012. PMID: 23226788 Free PMC article.
-
Kinesin family deregulation coordinated by bromodomain protein ANCCA and histone methyltransferase MLL for breast cancer cell growth, survival, and tamoxifen resistance.Mol Cancer Res. 2014 Apr;12(4):539-49. doi: 10.1158/1541-7786.MCR-13-0459. Epub 2014 Jan 3. Mol Cancer Res. 2014. PMID: 24391143 Free PMC article.
-
Steroid Receptor Coactivator-3 (SRC-3/AIB1) as a Novel Therapeutic Target in Triple Negative Breast Cancer and Its Inhibition with a Phospho-Bufalin Prodrug.PLoS One. 2015 Oct 2;10(10):e0140011. doi: 10.1371/journal.pone.0140011. eCollection 2015. PLoS One. 2015. PMID: 26431029 Free PMC article.
-
Molecular Mechanisms of Endocrine Resistance in Estrogen-Positive Breast Cancer.Front Endocrinol (Lausanne). 2021 Mar 25;12:599586. doi: 10.3389/fendo.2021.599586. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33841325 Free PMC article. Review.
References
-
- Al-Dhaheri MH, Rowan BG. Protein kinase A exhibits selective modulation of estradiol dependent transcription in breast cancer cells that is associated with decreased ligand binding, altered estrogen receptor alpha promoter interaction and changes in receptor phosphorylation. Mol Endocrinol. 2006;21:439–456. - PubMed
-
- Arteaga CL, Koli KM, Dugger TC, Clarke R. Reversal of tamoxifen resistance of human breast carcinomas in vivo by neuralizing antibodies to transforming growth factor-β. J Natl Cancer Inst. 1999;91:46–53. - PubMed
-
- Brunner N, Boulay V, Fojo A, Freter CE, Lippman ME, Clarke R. Acquisition of hormone-independent growth in MCF-7 cells is accompanied by increased expression of estrogen-regulated genes but without detectable DNA amplifications. Cancer Research. 1993a;53:283–290. - PubMed
-
- Brunner N, Frandsen TL, Holst-Hansen C, Bei M, Thompson EW, Wakeling AE, Lippman ME, Clarke R. MCF7/LCC2: a 4-hydroxytamoxifen resistant human breast cancer variant that retains sensitivity to the steroidal antiestrogen ICI 182,780. Cancer Research. 1993b;53:3229–3232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

