Human tumor virus utilizes exosomes for intercellular communication
- PMID: 21059916
- PMCID: PMC2996715
- DOI: 10.1073/pnas.1014194107
Human tumor virus utilizes exosomes for intercellular communication
Abstract
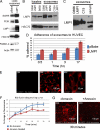
The Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) is expressed in multiple human malignancies and has potent effects on cell growth. It has been detected in exosomes and shown to inhibit immune function. Exosomes are small secreted cellular vesicles that contain proteins, mRNAs, and microRNAs (miRNAs). When produced by malignant cells, they can promote angiogenesis, cell proliferation, tumor-cell invasion, and immune evasion. In this study, exosomes released from nasopharyngeal carcinoma (NPC) cells harboring latent EBV were shown to contain LMP1, signal transduction molecules, and virus-encoded miRNAs. Exposure to these NPC exosomes activated the ERK and AKT signaling pathways in the recipient cells. Interestingly, NPC exosomes also contained viral miRNAs, several of which were enriched in comparison with their intracellular levels. LMP1 induces expression of the EGF receptor in an EBV-negative epithelial cell line, and exosomes produced by these cells also contain high levels of EGF receptor in exosomes. These findings suggest that the effects of EBV and LMP1 on cellular expression also modulate exosome content and properties. The exosomes may manipulate the tumor microenvironment to influence the growth of neighboring cells through the intercellular transfer of LMP1, signaling molecules, and viral miRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Raab-Traub N. Epstein–Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431–441. - PubMed
-
- Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. - PubMed
-
- Morris MA, Dawson CW, Young LS. Role of the Epstein–Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncol. 2009;5:811–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

