Molecular chaperone function of Mia40 triggers consecutive induced folding steps of the substrate in mitochondrial protein import
- PMID: 21059946
- PMCID: PMC2996643
- DOI: 10.1073/pnas.1010095107
Molecular chaperone function of Mia40 triggers consecutive induced folding steps of the substrate in mitochondrial protein import
Abstract
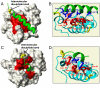
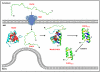
Several proteins of the mitochondrial intermembrane space are targeted by internal targeting signals. A class of such proteins with α-helical hairpin structure bridged by two intramolecular disulfides is trapped by a Mia40-dependent oxidative process. Here, we describe the oxidative folding mechanism underpinning this process by an exhaustive structural characterization of the protein in all stages and as a complex with Mia40. Two consecutive induced folding steps are at the basis of the protein-trapping process. In the first one, Mia40 functions as a molecular chaperone assisting α-helical folding of the internal targeting signal of the substrate. Subsequently, in a Mia40-independent manner, folding of the second substrate helix is induced by the folded targeting signal functioning as a folding scaffold. The Mia40-induced folding pathway provides a proof of principle for the general concept that internal targeting signals may operate as a folding nucleus upon compartment-specific activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The mitochondrial intermembrane space oxireductase Mia40 funnels the oxidative folding pathway of the cytochrome c oxidase assembly protein Cox19.J Biol Chem. 2014 Apr 4;289(14):9852-64. doi: 10.1074/jbc.M114.553479. Epub 2014 Feb 25. J Biol Chem. 2014. PMID: 24569988 Free PMC article.
-
The MIA pathway: a key regulator of mitochondrial oxidative protein folding and biogenesis.Acc Chem Res. 2015 Aug 18;48(8):2191-9. doi: 10.1021/acs.accounts.5b00150. Epub 2015 Jul 27. Acc Chem Res. 2015. PMID: 26214018 Free PMC article.
-
A novel intermembrane space-targeting signal docks cysteines onto Mia40 during mitochondrial oxidative folding.J Cell Biol. 2009 Dec 28;187(7):1007-22. doi: 10.1083/jcb.200905134. Epub 2009 Dec 21. J Cell Biol. 2009. PMID: 20026652 Free PMC article.
-
A disulfide relay system in mitochondria.Cell. 2005 Jul 1;121(7):965-7. doi: 10.1016/j.cell.2005.06.019. Cell. 2005. PMID: 15989945 Review.
-
Oxidative protein folding in the mitochondrial intermembrane space.Antioxid Redox Signal. 2010 Oct;13(8):1189-204. doi: 10.1089/ars.2010.3157. Antioxid Redox Signal. 2010. PMID: 20214493 Review.
Cited by
-
Decreased expression of the augmenter of liver regeneration results in increased apoptosis and oxidative damage in human-derived glioma cells.Cell Death Dis. 2012 Apr 5;3(4):e289. doi: 10.1038/cddis.2012.25. Cell Death Dis. 2012. PMID: 22476097 Free PMC article.
-
Mitochondrial translocation of APE1 relies on the MIA pathway.Nucleic Acids Res. 2015 Jun 23;43(11):5451-64. doi: 10.1093/nar/gkv433. Epub 2015 May 8. Nucleic Acids Res. 2015. PMID: 25956655 Free PMC article.
-
Proteasomal degradation induced by DPP9-mediated processing competes with mitochondrial protein import.EMBO J. 2020 Oct 1;39(19):e103889. doi: 10.15252/embj.2019103889. Epub 2020 Aug 20. EMBO J. 2020. PMID: 32815200 Free PMC article.
-
The mitochondrial intermembrane space oxireductase Mia40 funnels the oxidative folding pathway of the cytochrome c oxidase assembly protein Cox19.J Biol Chem. 2014 Apr 4;289(14):9852-64. doi: 10.1074/jbc.M114.553479. Epub 2014 Feb 25. J Biol Chem. 2014. PMID: 24569988 Free PMC article.
-
Building the CuA site of cytochrome c oxidase: A complicated, redox-dependent process driven by a surprisingly large complement of accessory proteins.J Biol Chem. 2018 Mar 30;293(13):4644-4652. doi: 10.1074/jbc.R117.816132. Epub 2017 Sep 29. J Biol Chem. 2018. PMID: 28972150 Free PMC article. Review.
References
-
- Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. - PubMed
-
- Lu H, et al. Functional TIM10 chaperone assembly is redox-regulated in vivo. J Biol Chem. 2004;279:18952–18958. - PubMed
-
- Webb CT, et al. Crystal structure of the mitochondrial chaperone TIM9.10 reveals a six-bladed alpha-propeller. Mol Cell. 2006;21:123–133. - PubMed
-
- Banci L, et al. A structural-dynamical characterization of human Cox17. J Biol Chem. 2008;283:7912–7920. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

