CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells
- PMID: 21059953
- PMCID: PMC2996674
- DOI: 10.1073/pnas.1012865107
CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells
Abstract
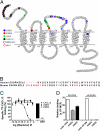
The important family of G protein-coupled receptors has so far not been targeted very successfully with conventional monoclonal antibodies. Here we report the isolation and characterization of functional VHH-based immunoglobulin single variable domains (or nanobodies) against the chemokine receptor CXCR4. Two highly selective monovalent nanobodies, 238D2 and 238D4, were obtained using a time-efficient whole cell immunization, phage display, and counterselection method. The highly selective VHH-based immunoglobulin single variable domains competitively inhibited the CXCR4-mediated signaling and antagonized the chemoattractant effect of the CXCR4 ligand CXCL12. Epitope mapping showed that the two nanobodies bind to distinct but partially overlapping sites in the extracellular loops. Short peptide linkage of 238D2 with 238D4 resulted in significantly increased affinity for CXCR4 and picomolar activity in antichemotactic assays. Interestingly, the monovalent nanobodies behaved as neutral antagonists, whereas the biparatopic nanobodies acted as inverse agonists at the constitutively active CXCR4-N3.35A. The CXCR4 nanobodies displayed strong antiretroviral activity against T cell-tropic and dual-tropic HIV-1 strains. Moreover, the biparatopic nanobody effectively mobilized CD34-positive stem cells in cynomolgus monkeys. Thus, the nanobody platform may be highly effective at generating extremely potent and selective G protein-coupled receptor modulators.
Conflict of interest statement
Conflict of interest statement: M.G.-P., S.D.V., B.S., H.U., W.V., M.S., H.J.D.H., and P.V. are/were paid employees of Ablynx.
Figures
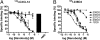
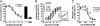


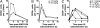
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

