Temporoparietal hypometabolism in frontotemporal lobar degeneration and associated imaging diagnostic errors
- PMID: 21059987
- PMCID: PMC3058918
- DOI: 10.1001/archneurol.2010.295
Temporoparietal hypometabolism in frontotemporal lobar degeneration and associated imaging diagnostic errors
Abstract
Objective: To evaluate the cause of diagnostic errors in the visual interpretation of positron emission tomographic scans with fludeoxyglucose F 18 (FDG-PET) in patients with frontotemporal lobar degeneration (FTLD) and patients with Alzheimer disease (AD).
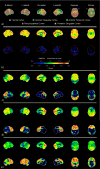
Design: Twelve trained raters unaware of clinical and autopsy information independently reviewed FDG-PET scans and provided their diagnostic impression and confidence of either FTLD or AD. Six of these raters also recorded whether metabolism appeared normal or abnormal in 5 predefined brain regions in each hemisphere-frontal cortex, anterior cingulate cortex, anterior temporal cortex, temporoparietal cortex, and posterior cingulate cortex. Results were compared with neuropathological diagnoses.
Setting: Academic medical centers.
Patients: Forty-five patients with pathologically confirmed FTLD (n=14) or AD (n=31).
Results: Raters had a high degree of diagnostic accuracy in the interpretation of FDG-PET scans; however, raters consistently found some scans more difficult to interpret than others. Unanimity of diagnosis among the raters was more frequent in patients with AD (27 of 31 patients [87%]) than in patients with FTLD (7 of 14 patients [50%]) (P=.02). Disagreements in interpretation of scans in patients with FTLD largely occurred when there was temporoparietal hypometabolism, which was present in 7 of the 14 FTLD scans and 6 of the 7 scans lacking unanimity. Hypometabolism of anterior cingulate and anterior temporal regions had higher specificities and positive likelihood ratios for FTLD than temporoparietal hypometabolism had for AD.
Conclusions: Temporoparietal hypometabolism in FTLD is common and may cause inaccurate interpretation of FDG-PET scans. An interpretation paradigm that focuses on the absence of hypometabolism in regions typically affected in AD before considering FTLD is likely to misclassify a significant portion of FTLD scans. Anterior cingulate and/or anterior temporal hypometabolism indicates a high likelihood of FTLD, even when temporoparietal hypometabolism is present. Ultimately, the accurate interpretation of FDG-PET scans in patients with dementia cannot rest on the presence or absence of a single region of hypometabolism but rather must take into account the relative hypometabolism of all brain regions.
Figures

Similar articles
-
Diagnostic Accuracy of Amyloid versus 18 F-Fluorodeoxyglucose Positron Emission Tomography in Autopsy-Confirmed Dementia.Ann Neurol. 2021 Feb;89(2):389-401. doi: 10.1002/ana.25968. Epub 2020 Dec 7. Ann Neurol. 2021. PMID: 33219525 Free PMC article.
-
A visual [18F]FDG-PET rating scale for the differential diagnosis of frontotemporal lobar degeneration.Eur Arch Psychiatry Clin Neurosci. 2011 Sep;261(6):433-46. doi: 10.1007/s00406-010-0184-0. Epub 2011 Jan 5. Eur Arch Psychiatry Clin Neurosci. 2011. PMID: 21207049
-
Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD.Neurology. 2011 Dec 6;77(23):2034-42. doi: 10.1212/WNL.0b013e31823b9c5e. Epub 2011 Nov 30. Neurology. 2011. PMID: 22131541 Free PMC article.
-
Brain fluorodeoxyglucose (FDG) PET in dementia.Ageing Res Rev. 2016 Sep;30:73-84. doi: 10.1016/j.arr.2016.02.003. Epub 2016 Feb 11. Ageing Res Rev. 2016. PMID: 26876244 Review.
-
Molecular and Anatomical Imaging of Dementia With Lewy Bodies and Frontotemporal Lobar Degeneration.Semin Nucl Med. 2021 May;51(3):264-274. doi: 10.1053/j.semnuclmed.2020.12.002. Epub 2021 Jan 2. Semin Nucl Med. 2021. PMID: 33402272 Review.
Cited by
-
Neuroimaging in frontotemporal lobar degeneration--predicting molecular pathology.Nat Rev Neurol. 2012 Jan 31;8(3):131-42. doi: 10.1038/nrneurol.2012.7. Nat Rev Neurol. 2012. PMID: 22290573 Review.
-
Mapping neuroinflammation in frontotemporal dementia with molecular PET imaging.J Neuroinflammation. 2015 May 29;12:108. doi: 10.1186/s12974-015-0236-5. J Neuroinflammation. 2015. PMID: 26022249 Free PMC article. Review.
-
Current Role for Biomarkers in Clinical Diagnosis of Alzheimer Disease and Frontotemporal Dementia.Curr Treat Options Neurol. 2017 Nov 14;19(12):46. doi: 10.1007/s11940-017-0484-z. Curr Treat Options Neurol. 2017. PMID: 29134465 Review.
-
Unraveling the interplay between sleep, redox metabolism, and aging: implications for brain health and longevity.Front Aging. 2025 May 21;6:1605070. doi: 10.3389/fragi.2025.1605070. eCollection 2025. Front Aging. 2025. PMID: 40469623 Free PMC article. Review.
-
Multiplex digital spatial profiling identifies subregion dependent targeted proteome changes across variants of dementia.NPJ Dement. 2025;1(1):10. doi: 10.1038/s44400-025-00010-6. Epub 2025 Jun 3. NPJ Dement. 2025. PMID: 40476255 Free PMC article.
References
-
- Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002 Oct-Dec;16(4):203–212. - PubMed
-
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998 Dec;51(6):1546–1554. - PubMed
-
- Baker M, Mackenzie I, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006 Aug 24;442(7105):916–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG028377/AG/NIA NIH HHS/United States
- P50 AG008671/AG/NIA NIH HHS/United States
- AG16573/AG/NIA NIH HHS/United States
- AG08671/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- AG05133/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- AG028377/AG/NIA NIH HHS/United States
- AG30006/AG/NIA NIH HHS/United States
- AG10129/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- AG10124/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- K23 AG030006/AG/NIA NIH HHS/United States
- AG12300/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- R01 AG022394/AG/NIA NIH HHS/United States
- AG16976/AG/NIA NIH HHS/United States
- AG10133/AG/NIA NIH HHS/United States
- AG22394/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States

