Pulmonary hypertension-induced GATA4 activation in the right ventricle
- PMID: 21059997
- PMCID: PMC4157308
- DOI: 10.1161/HYPERTENSIONAHA.110.160515
Pulmonary hypertension-induced GATA4 activation in the right ventricle
Abstract
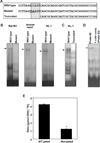
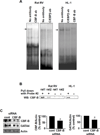
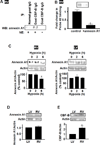
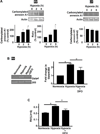
The major cause of death among pulmonary hypertension patients is right heart failure, but the biology of right heart is not well understood. Previous studies showed that mechanisms of the activation of GATA4, a major regulator of cardiac hypertrophy, in response to pressure overload are different between left and right ventricles. In the left ventricle, aortic constriction triggers GATA4 activation via posttranslational modifications without influencing GATA4 expression, while pulmonary artery banding enhances GATA4 expression in the right ventricle. We found that GATA4 expression can also be increased in the right ventricle of rats treated with chronic hypoxia to induce pulmonary hypertension and investigated the mechanism of increased GATA4 expression. Examination of Gata4 promoter revealed that CCAAT box plays an important role in gene activation, and hypoxic pulmonary hypertension promoted the binding of CCAAT-binding factor/nuclear factor-Y (CBF/NF-Y) to CCAAT box in the right ventricle. We found that CBF/NF-Y forms a complex with annexin A1, which inhibits DNA binding activity. In response to hypoxic pulmonary hypertension, annexin A1 gets degraded, resulting in CBF/NF-Y-dependent activation of Gata4 gene transcription. The right ventricle contains a higher level of CBF/NF-Y compared to the left ventricle, and this may allow for efficient activation in response to annexin A1 degradation. Signaling via iron-catalyzed protein oxidation mediates hypoxic pulmonary hypertension-induced annexin A1 degradation, Gata4 gene transcription, and right ventricular hypertrophy. These results establish a right heart-specific signaling mechanism in response to pressure overload, which involves metal-catalyzed carbonylation and degradation of annexin A1 that liberates CBF/NF-Y to activate Gata4 gene transcription.
Conflict of interest statement
None.
Figures


Similar articles
-
Redox Signaling in the Right Ventricle.Adv Exp Med Biol. 2017;967:315-323. doi: 10.1007/978-3-319-63245-2_19. Adv Exp Med Biol. 2017. PMID: 29047095
-
Mechanism of anthracycline-mediated down-regulation of GATA4 in the heart.Cardiovasc Res. 2011 Apr 1;90(1):97-104. doi: 10.1093/cvr/cvq361. Epub 2010 Nov 16. Cardiovasc Res. 2011. PMID: 21084315 Free PMC article.
-
Differential responses of the right ventricle to abnormal loading conditions in vivo: possible pathophysiologic mechanisms.J Thorac Cardiovasc Surg. 2013 May;145(5):1335-44. doi: 10.1016/j.jtcvs.2013.01.016. Epub 2013 Feb 20. J Thorac Cardiovasc Surg. 2013. PMID: 23434296
-
Right ventricle in pulmonary hypertension.Compr Physiol. 2011 Jan;1(1):525-40. doi: 10.1002/cphy.c090008. Compr Physiol. 2011. PMID: 23737184 Review.
-
[Development of Targeted Pharmacotherapy for Cardiovascular Disease].Yakugaku Zasshi. 2017;137(11):1349-1353. doi: 10.1248/yakushi.17-00171. Yakugaku Zasshi. 2017. PMID: 29093370 Review. Japanese.
Cited by
-
Pressure-overload-induced right heart failure.Pflugers Arch. 2014 Jun;466(6):1055-63. doi: 10.1007/s00424-014-1450-1. Epub 2014 Feb 1. Pflugers Arch. 2014. PMID: 24488007 Review.
-
Cell signaling pathways for the regulation of GATA4 transcription factor: Implications for cell growth and apoptosis.Cell Signal. 2011 Jul;23(7):1094-9. doi: 10.1016/j.cellsig.2011.02.007. Epub 2011 Mar 1. Cell Signal. 2011. PMID: 21376121 Free PMC article.
-
Hemodynamic response to treatment of iron deficiency anemia in pulmonary arterial hypertension: longitudinal insights from an implantable hemodynamic monitor.Pulm Circ. 2016 Dec;6(4):616-618. doi: 10.1086/688670. Pulm Circ. 2016. PMID: 28090307 Free PMC article.
-
The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth.Br J Pharmacol. 2013 Jan;168(2):296-317. doi: 10.1111/j.1476-5381.2012.02195.x. Br J Pharmacol. 2013. PMID: 22946456 Free PMC article. Review.
-
Cardiomyocyte-specific overexpression of HEXIM1 prevents right ventricular hypertrophy in hypoxia-induced pulmonary hypertension in mice.PLoS One. 2012;7(12):e52522. doi: 10.1371/journal.pone.0052522. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300697 Free PMC article.
References
-
- Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. - PubMed
-
- Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and-6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–38952. - PubMed
-
- Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem. 2001;276:30245–30253. - PubMed
-
- Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

