Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954
- PMID: 21060024
- PMCID: PMC3020693
- DOI: 10.1200/JCO.2009.26.6114
Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954
Abstract
Purpose: Patients with locally advanced gastric cancer benefit from combined pre- and postoperative chemotherapy, although fewer than 50% could receive postoperative chemotherapy. We examined the value of purely preoperative chemotherapy in a phase III trial with strict preoperative staging and surgical resection guidelines.
Patients and methods: Patients with locally advanced adenocarcinoma of the stomach or esophagogastric junction (AEG II and III) were randomly assigned to preoperative chemotherapy followed by surgery or to surgery alone. To detect with 80% power an improvement in median survival from 17 months with surgery alone to 24 months with neoadjuvant, 282 events were required.
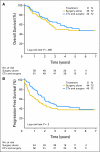
Results: This trial was stopped for poor accrual after 144 patients were randomly assigned (72:72); 52.8% patients had tumors located in the proximal third of the stomach, including AEG type II and III. The International Union Against Cancer R0 resection rate was 81.9% after neoadjuvant chemotherapy as compared with 66.7% with surgery alone (P = .036). The surgery-only group had more lymph node metastases than the neoadjuvant group (76.5% v 61.4%; P = .018). Postoperative complications were more frequent in the neoadjuvant arm (27.1% v 16.2%; P = .09). After a median follow-up of 4.4 years and 67 deaths, a survival benefit could not be shown (hazard ratio, 0.84; 95% CI, 0.52 to 1.35; P = .466).
Conclusion: This trial showed a significantly increased R0 resection rate but failed to demonstrate a survival benefit. Possible explanations are low statistical power, a high rate of proximal gastric cancer including AEG and/or a better outcome than expected after radical surgery alone due to the high quality of surgery with resections of regional lymph nodes outside the perigastic area (celiac trunc, hepatic ligament, lymph node at a. lienalis; D2).
Trial registration: ClinicalTrials.gov NCT00004099.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer: Pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–2403. - PubMed
-
- Greenlee RT, Murray T, Bolden S, et al. Cancer statistics, 2000. Cancer J Clin. 2000;50:7–33. - PubMed
-
- Roder JD, Böttcher K, Siewert JR. Prognostic factors in gastric carcinoma: Results of the German Gastric Carcinoma study 1992. Cancer. 1993;72:2089–2097. - PubMed
-
- Paoletti X, Oba K, Burzykowski T, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. JAMA. 2010;303:1729–1737. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical