Accrual experience of National Cancer Institute Cooperative Group phase III trials activated from 2000 to 2007
- PMID: 21060029
- PMCID: PMC3020692
- DOI: 10.1200/JCO.2010.31.5382
Accrual experience of National Cancer Institute Cooperative Group phase III trials activated from 2000 to 2007
Abstract
Purpose: Recent reports have suggested that 40% or more of National Cancer Institute (NCI) -sponsored Cooperative Group phase III trials failed to achieve their accrual goals. We examine in detail the accrual experience of the Cooperative Group phase III trials.
Patients and methods: All Cooperative Group phase III trials activated from 2000 to 2007 were examined for their accrual experience. For trials that stopped accrual with < 90% of their accrual goal, the reasons for having < 90% accrual were documented. We focus on trials that ended with < 90% accrual because of inadequate accrual rates rather than for other reasons, such as an interim monitoring analysis by an independent data monitoring committee that stops the trial early because one treatment is clearly superior.
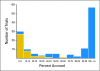
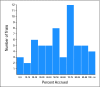
Results: There were 191 trials activated from 2000 to 2007. We project that 22.0% of these trials will have < 90% accrual because of inadequate accrual rates. We project that there will be 176,627 patients eventually accrued on the 191 trials (current accrual, 154,579) and that 2,991 of these patients will be on trials that have < 90% accrual because of inadequate accrual rates (1.7%). For nonpediatric cancer trials, the corresponding percentages are 26.7% and 2.0%.
Conclusion: We find that insufficient accrual rates are not as high as previously reported and that only a small proportion of patients were enrolled on trials that ended with insufficient accrual because of an inadequate accrual rate. NCI has implemented new procedures to reduce the number of trials that fail to reach their accrual goals and to minimize the number of patients accrued on these trials.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Zuckerman B, Corrigan J. The role of NCI-sponsored research in ASCO Clinical Cancer Advances 2005-2008. J Clin Oncol. 2010;28(suppl):470s. abstr 6094.
-
- Korn EL, Dorey FJ. Applications of crude incidence curves. Stat Med. 1992;11:813–829. - PubMed
-
- Institute of Medicine. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: The National Academies Press; 2010. p. 143. - PubMed
-
- Editorial: Faltering cancer trials. New York Times, April 24, 2010 (a version of this editorial appeared on p WK11 of the New York edition on April, 25, 2010) http://www.nytimes.com/2010/04/25/opinion/25sun1.html.
MeSH terms
LinkOut - more resources
Full Text Sources