Identification and characterization of persistent intracellular human immunodeficiency virus type 1 integrase strand transfer inhibitor activity
- PMID: 21060108
- PMCID: PMC3019619
- DOI: 10.1128/AAC.01064-10
Identification and characterization of persistent intracellular human immunodeficiency virus type 1 integrase strand transfer inhibitor activity
Abstract
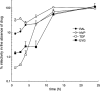
Pharmacokinetic and pharmacodynamic considerations significantly impact infectious disease treatment options. One aspect of pharmacodynamics is the postantibiotic effect, classically defined as delayed bacterial growth after antibiotic removal. The same principle can apply to antiviral drugs. For example, significant delays in human immunodeficiency virus type 1 (HIV-1) replication can be observed after nucleoside/nucleotide reverse transcriptase inhibitor (N/NtRTI) removal from culture medium, because these prodrugs must be anabolized into active, phosphorylated forms once internalized into cells. A relatively new class of anti-HIV-1 drugs is the integrase strand transfer inhibitors (INSTIs), and the INSTIs raltegravir (RAL) and elvitegravir (EVG) were tested here alongside positive N/NtRTI controls tenofovir disoproxil fumarate (TDF) and azidothymidine (AZT), as well as the nonnucleoside reverse transcriptase inhibitor negative control nevirapine (NVP), to assess potential postantiviral effects. Transformed and primary CD4-positive cells pretreated with INSTIs significantly resisted subsequent challenge by HIV-1, revealing the following hierarchy of persistent intracellular drug strength: TDF > EVG ∼ AZT > RAL > NVP. A modified time-of-addition assay was moreover developed to assess residual drug activity levels. Approximately 0.8% of RAL and 2% of initial EVG and TDF 1-h pulse drug levels persisted during the acute phase of HIV-1 infection. EVG furthermore displayed significant virucidal activity. Although there is no reason to suspect obligate intracellular modification, this study nevertheless defines significant intracellular persistence of prototype INSTIs. Ongoing second-generation formulations should therefore consider the potential for significant postantiviral effects among this drug class. Combined intracellular persistence and virucidal activities suggest potential pre-exposure prophylaxis applications for EVG.
Figures
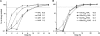

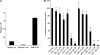
References
-
- Azoulay, S., M.-C. Nevers, C. Creminon, L. Heripret, J. Durant, P. Dellamonica, J. Grassi, R. Guedj, and D. Duval. 2004. Sensitive enzyme immunoassay for measuring plasma and intracellular nevirapine levels in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 48:104-109. - PMC - PubMed
-
- Balzarini, J., A. Holy, J. Jindrich, L. Naesens, R. Snoeck, D. Schols, and E. De Clercq. 1993. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 37:332-338. - PMC - PubMed
-
- Balzarini, J., L. Naesens, S. Aquaro, T. Knispel, C. Perno, E. De Clercq, and C. Meier. 1999. Intracellular metabolism of CycloSaligenyl 3′-azido-2′,3′-dideoxythymidine monophosphate, a prodrug of 3′-azido-2′,3′-dideoxythymidine (zidovudine). Mol. Pharmacol. 56:1354-1361. - PubMed
-
- Collman, R. 1992. Human immunodeficiency virus type 1 tropism for human macrophages. Pathobiology 60:213-218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

