The corticofugal neuron-associated genes ROBO1, SRGAP1, and CTIP2 exhibit an anterior to posterior gradient of expression in early fetal human neocortex development
- PMID: 21060114
- PMCID: PMC3097990
- DOI: 10.1093/cercor/bhq219
The corticofugal neuron-associated genes ROBO1, SRGAP1, and CTIP2 exhibit an anterior to posterior gradient of expression in early fetal human neocortex development
Abstract
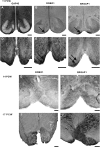
Developing neocortical progenitors express transcription factors in gradients that induce programs of region-specific gene expression. Our previous work identified anteriorly upregulated expression gradients of a number of corticofugal neuron-associated gene probe sets along the anterior-posterior axis of the human neocortex (8-12 postconceptional weeks [PCW]). Here, we demonstrate by real-time polymerase chain reaction, in situ hybridization and immunohistochemistry that 3 such genes, ROBO1, SRGAP1, and CTIP2 are highly expressed anteriorly between 8-12 PCW, in comparison with other genes (FEZF2, SOX5) expressed by Layer V, VI, and subplate neurons. All 3 were prominently expressed by early postmitotic neurons in the subventricular zone, intermediate zone, and cortical plate (CP) from 8 to 10 PCW. Between 12 and 15 PCW expression patterns for ER81 and SATB2 (Layer V), TBR1 (Layer V/VI) and NURR1 (Layer VI) revealed Layer V forming. By 15 PCW, ROBO1 and SRGAP1 expression was confined to Layer V, whereas CTIP2 was expressed throughout the CP anteriorly. We observed ROBO1 and SRGAP1 immunoreactivity in medullary corticospinal axons from 11 PCW onward. Thus, we propose that the coexpression of these 3 markers in the anterior neocortex may mark the early location of the human motor cortex, including its corticospinal projection neurons, allowing further study of their early differentiation.
Figures





References
-
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. - PubMed
-
- Andrews W, Barber M, Hernadez-Miranda LR, Xian J, Rakic S, Sundaresan V, Rabbitts TH, Pannell R, Rabbitts P, Thompson H, et al. The role of Slit-Robo signaling in the generation, migration and morphological differentiation of cortical interneurons. Dev Biol. 2008;313:648–658. - PubMed
-
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

