Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice
- PMID: 21060152
- PMCID: PMC2993576
- DOI: 10.1172/JCI36858
Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice
Abstract
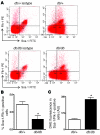
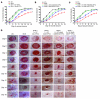
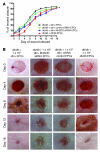
Amputation as a result of impaired wound healing is a serious complication of diabetes. Inadequate angiogenesis contributes to poor wound healing in diabetic patients. Endothelial progenitor cells (EPCs) normally augment angiogenesis and wound repair but are functionally impaired in diabetics. Here we report that decreased expression of manganese superoxide dismutase (MnSOD) in EPCs contributes to impaired would healing in a mouse model of type 2 diabetes. A decreased frequency of circulating EPCs was detected in type 2 diabetic (db/db) mice, and when isolated, these cells exhibited decreased expression and activity of MnSOD. Wound healing and angiogenesis were markedly delayed in diabetic mice compared with normal controls. For cell therapy, topical transplantation of EPCs onto excisional wounds in diabetic mice demonstrated that diabetic EPCs were less effective than normal EPCs at accelerating wound closure. Transplantation of diabetic EPCs after MnSOD gene therapy restored their ability to mediate angiogenesis and wound repair. Conversely, siRNA-mediated knockdown of MnSOD in normal EPCs reduced their activity in diabetic wound healing assays. Increasing the number of transplanted diabetic EPCs also improved the rate of wound closure. Our findings demonstrate that cell therapy using diabetic EPCs after ex vivo MnSOD gene transfer accelerates their ability to heal wounds in a mouse model of type 2 diabetes.
Figures







References
-
- Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence-based protocol for diabetic foot ulcers. Plast Reconstr Surg. 2006;117(7 suppl):193S–209S. - PubMed
-
- Loomans CJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53(1):195–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

