Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: occurrence and recent bioactivity studies
- PMID: 21060304
- PMCID: PMC6259451
- DOI: 10.3390/molecules15117985
Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: occurrence and recent bioactivity studies
Abstract
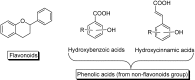
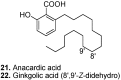
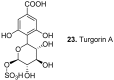
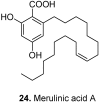
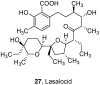
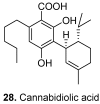
Among the wide diversity of naturally occurring phenolic acids, at least 30 hydroxy- and polyhydroxybenzoic acids have been reported in the last 10 years to have biological activities. The chemical structures, natural occurrence throughout the plant, algal, bacterial, fungal and animal kingdoms, and recently described bioactivities of these phenolic and polyphenolic acids are reviewed to illustrate their wide distribution, biological and ecological importance, and potential as new leads for the development of pharmaceutical and agricultural products to improve human health and nutrition.
Figures
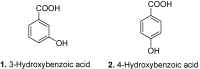

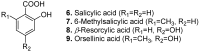





Similar articles
-
Structure-antioxidant activity relationships of flavonoids and phenolic acids.Free Radic Biol Med. 1996;20(7):933-56. doi: 10.1016/0891-5849(95)02227-9. Free Radic Biol Med. 1996. PMID: 8743980
-
Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship.Crit Rev Food Sci Nutr. 2004;44(4):253-73. doi: 10.1080/10408690490464960. Crit Rev Food Sci Nutr. 2004. PMID: 15462129 Review.
-
Defensive strategies in Geranium sylvaticum, Part 2: Roles of water-soluble tannins, flavonoids and phenolic acids against natural enemies.Phytochemistry. 2013 Nov;95:408-20. doi: 10.1016/j.phytochem.2013.07.029. Epub 2013 Sep 16. Phytochemistry. 2013. PMID: 24050514
-
Efficiency of trapping methylglyoxal by phenols and phenolic acids.J Food Sci. 2011 Apr;76(3):H90-6. doi: 10.1111/j.1750-3841.2011.02067.x. Epub 2011 Mar 16. J Food Sci. 2011. PMID: 21535836
-
Phenolic acids in cereal grain: Occurrence, biosynthesis, metabolism and role in living organisms.Crit Rev Food Sci Nutr. 2019;59(4):664-675. doi: 10.1080/10408398.2017.1387096. Epub 2017 Nov 20. Crit Rev Food Sci Nutr. 2019. PMID: 28976227 Review.
Cited by
-
Impact of Foliar Application of Amino Acids on Total Phenols, Phenolic Acids Content of Different Mints Varieties under the Field Condition.Plants (Basel). 2021 Mar 23;10(3):599. doi: 10.3390/plants10030599. Plants (Basel). 2021. PMID: 33806769 Free PMC article.
-
Identification and Quantification of a Phytotoxic Metabolite from Alternaria dauci.Molecules. 2020 Sep 2;25(17):4003. doi: 10.3390/molecules25174003. Molecules. 2020. PMID: 32887350 Free PMC article.
-
A Review on Antidiabetic Properties of Indian Mangrove Plants with Reference to Island Ecosystem.Evid Based Complement Alternat Med. 2019 Dec 5;2019:4305148. doi: 10.1155/2019/4305148. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31885647 Free PMC article. Review.
-
Phenolic responses of mountain crowberry (Empetrum nigrum ssp. hermaphroditum) to global climate change are compound specific and depend on grazing by reindeer (Rangifer tarandus).J Chem Ecol. 2013 Dec;39(11-12):1390-9. doi: 10.1007/s10886-013-0367-z. Epub 2013 Nov 28. J Chem Ecol. 2013. PMID: 24287946
-
From Threat to Opportunity: Harnessing the Invasive Carpobrotus edulis (L.) N.E.Br for Nutritional and Phytotherapeutic Valorization Amid Seasonal and Spatial Variability.Mar Drugs. 2023 Aug 1;21(8):436. doi: 10.3390/md21080436. Mar Drugs. 2023. PMID: 37623717 Free PMC article.
References
-
- Martin K.R., Appel C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Dietary Suppl. 2010;2:1–12.
-
- Harris C.S., Mo F., Migahed L., Chepelev L., Haddad P.S., Wright J.S., Willmore W.G., Arnason J.T., Bennett S.A.L. Plant phenolics regulate neoplastic cell growth and survival: a quantitative structure-activity and biochemical analysis. Can. J. Physiol. Pharmacol. 2007;85:1124–1138. doi: 10.1139/Y07-101. - DOI - PubMed
-
- Huang W.Y., Cai Y.Z., Zhang Y.B. Natural Phenolic Compounds From Medicinal Herbs and Dietary Plants: Potential Use for Cancer Prevention. Nutr. Cancer. 2010;62:1–20. - PubMed
-
- Liu R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004;134:3479S–3485S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

