Nuclear factor-Y (NF-Y) regulates transcription of mouse Dmrt7 gene by binding to tandem CCAAT boxes in its proximal promoter
- PMID: 21060727
- PMCID: PMC2974168
- DOI: 10.7150/ijbs.6.655
Nuclear factor-Y (NF-Y) regulates transcription of mouse Dmrt7 gene by binding to tandem CCAAT boxes in its proximal promoter
Abstract
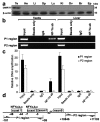
Dmrt7, a member of the Dmrt family of genes, is required for spermatogenesis. However, promoter functions of the gene Dmrt7 remain unknown. We have cloned and characterized the proximal promoter region of the mouse Dmrt7 gene. Functional analysis of the 5' flanking region by sequential deletion mutations revealed crucial positive elements between -60 and +1, in which two highly conserved and tandem CCAAT boxes: the CCAAT box1 (-48/-44) and the CCAAT box2 (-7/-3) are located. Site-directed mutagenesis studies demonstrated that both CCAAT boxes are indispensable to the promoter activity. Electrophoretic mobility shift assays (EMSAs) and gel-supershift assays indicated that transcription factor NF-Y binds to the promoter. Chromatin immunoprecipitation (ChIP) analysis demonstrated that NF-Y interacts in vivo with the promoter of the Dmrt7 gene in testis. Co-transfection and reporter analysis showed that over-expression of NF-Ys increased transcription of the Dmrt7-luc gene whereas expression of a dominant-negative NF-Ya decreased the transcription. This suggests that NF-Y can activate the Dmrt7 promoter. These results provide evidence of a transcription regulatory mechanism that controls Dmrt7 gene expression in mouse testis.
Keywords: DM domain; Endocrinology; Spermatogenesis; Transcriptional regulation.
Conflict of interest statement
Conflict of Interest: There are no conflicts of interest with the financing of this project and its subject matter.
Figures


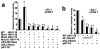
Similar articles
-
The CCAAT box binding transcription factor, nuclear factor-Y (NF-Y) regulates transcription of human aldo-keto reductase 1C1 (AKR1C1) gene.Gene. 2010 Jul 1;459(1-2):11-23. doi: 10.1016/j.gene.2010.03.006. Epub 2010 Mar 23. Gene. 2010. PMID: 20338228 Free PMC article.
-
Transcriptional regulation of the mouse PNRC2 promoter by the nuclear factor Y (NFY) and E2F1.Gene. 2005 Nov 21;361:89-100. doi: 10.1016/j.gene.2005.07.012. Epub 2005 Sep 21. Gene. 2005. PMID: 16181749
-
Oct-1 and nuclear factor Y bind to the SURG-1 element to direct basal and gonadotropin-releasing hormone (GnRH)-stimulated mouse GnRH receptor gene transcription.Mol Endocrinol. 2005 Jan;19(1):148-62. doi: 10.1210/me.2004-0025. Epub 2004 Sep 23. Mol Endocrinol. 2005. PMID: 15388790
-
Targeting the Y/CCAAT box in cancer: YB-1 (YBX1) or NF-Y?Cell Death Differ. 2013 May;20(5):676-85. doi: 10.1038/cdd.2013.13. Epub 2013 Mar 1. Cell Death Differ. 2013. PMID: 23449390 Free PMC article. Review.
-
A survey of 178 NF-Y binding CCAAT boxes.Nucleic Acids Res. 1998 Mar 1;26(5):1135-43. doi: 10.1093/nar/26.5.1135. Nucleic Acids Res. 1998. PMID: 9469818 Free PMC article. Review.
Cited by
-
Expression Analysis of IZUMO1 Gene during Testicular Development of Datong Yak (Bos Grunniens).Animals (Basel). 2019 May 29;9(6):292. doi: 10.3390/ani9060292. Animals (Basel). 2019. PMID: 31146500 Free PMC article.
-
Cloning, Molecular Characterization and Expression Patterns of DMRTC2 Implicated in Germ Cell Development of Male Tibetan Sheep.Int J Mol Sci. 2020 Apr 1;21(7):2448. doi: 10.3390/ijms21072448. Int J Mol Sci. 2020. PMID: 32244802 Free PMC article.
-
Expressional Profiling of TEX11, ESRα and BOLL Genes in Yak under Different Feeding Conditions.Biology (Basel). 2021 Jul 30;10(8):731. doi: 10.3390/biology10080731. Biology (Basel). 2021. PMID: 34439962 Free PMC article.
-
Morphometric Evaluation of Spermatogenic Cells and Seminiferous Tubules and Exploration of Luteinizing Hormone Beta Polypeptide in Testis of Datong Yak.Animals (Basel). 2019 Dec 30;10(1):66. doi: 10.3390/ani10010066. Animals (Basel). 2019. PMID: 31905946 Free PMC article.
-
Characterization of the distal promoter of the human pyruvate carboxylase gene in pancreatic beta cells.PLoS One. 2013;8(1):e55139. doi: 10.1371/journal.pone.0055139. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23383084 Free PMC article.
References
-
- Raymond CS, Shamu CE, Shen MM. et al.Evidence for evolutionary conservation of sex-determining genes. Nature. 1998;391(6668):691–695. - PubMed
-
- Zarkower D. Establishing sexual dimorphism: conservation amidst diversity? Nat Rev Genet. 2001;2(3):175–185. - PubMed
-
- Kondo M, Froschauer A, Kitano A. et al.Molecular cloning and characterization of DMRT genes from the medaka Oryzias latipes and the platyfish Xiphophorus maculatus. Gene. 2002;295(2):213–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

