Head-to-tail intramolecular interaction of herpes simplex virus type 1 regulatory protein ICP27 is important for its interaction with cellular mRNA export receptor TAP/NXF1
- PMID: 21060739
- PMCID: PMC2975367
- DOI: 10.1128/mBio.00268-10
Head-to-tail intramolecular interaction of herpes simplex virus type 1 regulatory protein ICP27 is important for its interaction with cellular mRNA export receptor TAP/NXF1
Abstract
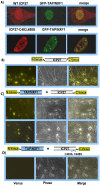
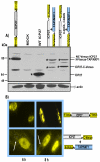
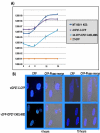
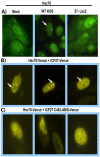
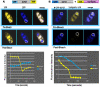
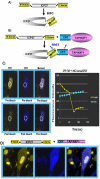
Herpes simplex virus type 1 (HSV-1) protein ICP27 has many important functions during infection that are achieved through interactions with a number of cellular proteins. In its role as a viral RNA export protein, ICP27 interacts with TAP/NXF1, the cellular mRNA export receptor, and both the N and C termini of ICP27 must be intact for this interaction to take place. Here we show by bimolecular fluorescence complementation (BiFC) that ICP27 interacts directly with TAP/NXF1 during infection, and this interaction failed to occur with an ICP27 mutant bearing substitutions of serines for cysteines at positions 483 and 488 in the C-terminal zinc finger. Recently, we showed that ICP27 undergoes a head-to-tail intramolecular interaction, which could make the N- and C-terminal regions accessible for binding to TAP/NXF1. To determine the importance of intramolecular association of ICP27 to its interaction with TAP/NXF1, we performed BiFC-based fluorescence resonance energy transfer (FRET) by acceptor photobleaching. BiFC-based FRET showed that the interaction between ICP27 and TAP/NXF1 occurred in living cells upon head-to-tail intramolecular association of ICP27, further establishing that TAP/NXF1 interacts with both the N and C termini of ICP27.
Figures


Similar articles
-
Herpes simplex virus 1 regulatory protein ICP27 undergoes a head-to-tail intramolecular interaction.J Virol. 2010 May;84(9):4124-35. doi: 10.1128/JVI.02319-09. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164236 Free PMC article.
-
The cellular RNA export receptor TAP/NXF1 is required for ICP27-mediated export of herpes simplex virus 1 RNA, but the TREX complex adaptor protein Aly/REF appears to be dispensable.J Virol. 2009 Jul;83(13):6335-46. doi: 10.1128/JVI.00375-09. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369354 Free PMC article.
-
ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export.J Virol. 2005 Apr;79(7):3949-61. doi: 10.1128/JVI.79.7.3949-3961.2005. J Virol. 2005. PMID: 15767397 Free PMC article.
-
The many roles of the regulatory protein ICP27 during herpes simplex virus infection.Front Biosci. 2008 May 1;13:5241-56. doi: 10.2741/3078. Front Biosci. 2008. PMID: 18508584 Review.
-
The many roles of the highly interactive HSV protein ICP27, a key regulator of infection.Future Microbiol. 2011 Nov;6(11):1261-77. doi: 10.2217/fmb.11.119. Future Microbiol. 2011. PMID: 22082288 Review.
Cited by
-
Current approaches on viral infection: proteomics and functional validations.Front Microbiol. 2012 Nov 16;3:393. doi: 10.3389/fmicb.2012.00393. eCollection 2012. Front Microbiol. 2012. PMID: 23162545 Free PMC article.
-
Monitoring the interactions between alpha-synuclein and Tau in vitro and in vivo using bimolecular fluorescence complementation.Sci Rep. 2022 Feb 22;12(1):2987. doi: 10.1038/s41598-022-06846-9. Sci Rep. 2022. PMID: 35194057 Free PMC article.
-
Nuclear export of plant pararetrovirus mRNAs involves the TREX complex, two viral proteins and the highly structured 5' leader region.Nucleic Acids Res. 2021 Sep 7;49(15):8900-8922. doi: 10.1093/nar/gkab653. Nucleic Acids Res. 2021. PMID: 34370034 Free PMC article.
-
Molecular Mechanism of SR Protein Kinase 1 Inhibition by the Herpes Virus Protein ICP27.mBio. 2019 Oct 22;10(5):e02551-19. doi: 10.1128/mBio.02551-19. mBio. 2019. PMID: 31641093 Free PMC article.
-
Stability of structured Kaposi's sarcoma-associated herpesvirus ORF57 protein is regulated by protein phosphorylation and homodimerization.J Virol. 2015 Mar;89(6):3256-74. doi: 10.1128/JVI.03721-14. Epub 2015 Jan 7. J Virol. 2015. PMID: 25568207 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
