Head-to-tail intramolecular interaction of herpes simplex virus type 1 regulatory protein ICP27 is important for its interaction with cellular mRNA export receptor TAP/NXF1
- PMID: 21060739
- PMCID: PMC2975367
- DOI: 10.1128/mBio.00268-10
Head-to-tail intramolecular interaction of herpes simplex virus type 1 regulatory protein ICP27 is important for its interaction with cellular mRNA export receptor TAP/NXF1
Abstract
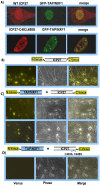
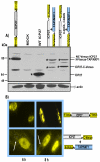
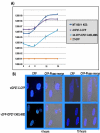
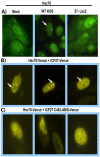
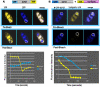
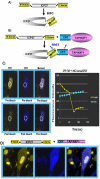
Herpes simplex virus type 1 (HSV-1) protein ICP27 has many important functions during infection that are achieved through interactions with a number of cellular proteins. In its role as a viral RNA export protein, ICP27 interacts with TAP/NXF1, the cellular mRNA export receptor, and both the N and C termini of ICP27 must be intact for this interaction to take place. Here we show by bimolecular fluorescence complementation (BiFC) that ICP27 interacts directly with TAP/NXF1 during infection, and this interaction failed to occur with an ICP27 mutant bearing substitutions of serines for cysteines at positions 483 and 488 in the C-terminal zinc finger. Recently, we showed that ICP27 undergoes a head-to-tail intramolecular interaction, which could make the N- and C-terminal regions accessible for binding to TAP/NXF1. To determine the importance of intramolecular association of ICP27 to its interaction with TAP/NXF1, we performed BiFC-based fluorescence resonance energy transfer (FRET) by acceptor photobleaching. BiFC-based FRET showed that the interaction between ICP27 and TAP/NXF1 occurred in living cells upon head-to-tail intramolecular association of ICP27, further establishing that TAP/NXF1 interacts with both the N and C termini of ICP27.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

