Exendin-4 protects oxidative stress-induced β-cell apoptosis through reduced JNK and GSK3β activity
- PMID: 21060752
- PMCID: PMC2967000
- DOI: 10.3346/jkms.2010.25.11.1626
Exendin-4 protects oxidative stress-induced β-cell apoptosis through reduced JNK and GSK3β activity
Abstract
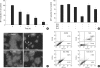
Oxidative stress induced by chronic hyperglycemia in type 2 diabetes plays a crucial role in progressive loss of β-cell mass through β-cell apoptosis. Glucagon like peptide-1 (GLP-1) has effects on preservation of β-cell mass and its insulin secretory function. GLP-1 possibly increases islet cell mass through stimulated proliferation from β-cell and differentiation to β-cell from progenitor cells. Also, it probably has an antiapoptotic effect on β-cell, but detailed mechanisms are not proven. Therefore, we examined the protective mechanism of GLP-1 in β-cell after induction of oxidative stress. The cell apoptosis decreased to ~50% when cells were treated with 100 µM H(2)O(2) for up to 2 hr. After pretreatment of Ex-4, GLP-1 receptor agonist, flow cytometric analysis shows 41.7% reduction of β-cell apoptosis. This data suggested that pretreatment of Ex-4 protect from oxidative stress-induced apoptosis. Also, Ex-4 treatment decreased GSK3β activation, JNK phosphorylation and caspase-9, -3 activation and recovered the expression of insulin2 mRNA in β-cell lines and secretion of insulin in human islet. These results suggest that Ex-4 may protect β-cell apoptosis by blocking the JNK and GSK3β mediated apoptotic pathway.
Keywords: Apoptosis; Exendin-4; Glucagon-Like Peptide 1; Insulin-Secreting Cells; Oxidative Stress.
Figures
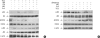
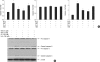
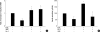
References
-
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patanè G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274:14112–14121. - PubMed
-
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. - PubMed
-
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

