Anti-angiogenic therapy induces integrin-linked kinase 1 up-regulation in a mouse model of glioblastoma
- PMID: 21060779
- PMCID: PMC2966411
- DOI: 10.1371/journal.pone.0013710
Anti-angiogenic therapy induces integrin-linked kinase 1 up-regulation in a mouse model of glioblastoma
Abstract
Background: In order to improve our understanding of the molecular pathways that mediate tumor proliferation and angiogenesis, and to evaluate the biological response to anti-angiogenic therapy, we analyzed the changes in the protein profile of glioblastoma in response to treatment with recombinant human Platelet Factor 4-DLR mutated protein (PF4-DLR), an inhibitor of angiogenesis.
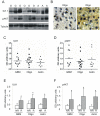
Methodology/principal findings: U87-derived experimental glioblastomas were grown in the brain of xenografted nude mice, treated with PF4-DLR, and processed for proteomic analysis. More than fifty proteins were differentially expressed in response to PF4-DLR treatment. Among them, integrin-linked kinase 1 (ILK1) signaling pathway was first down-regulated but then up-regulated after treatment for prolonged period. The activity of PF4-DLR can be increased by simultaneously treating mice orthotopically implanted with glioblastomas, with ILK1-specific siRNA. As ILK1 is related to malignant progression and a poor prognosis in various types of tumors, we measured ILK1 expression in human glioblastomas, astrocytomas and oligodendrogliomas, and found that it varied widely; however, a high level of ILK1 expression was correlated to a poor prognosis.
Conclusions/significance: Our results suggest that identifying the molecular pathways induced by anti-angiogenic therapies may help the development of combinatorial treatment strategies that increase the therapeutic efficacy of angiogenesis inhibitors by association with specific agents that disrupt signaling in tumor cells.
Conflict of interest statement
Figures


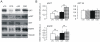


Similar articles
-
Prognostic significance of integrin-linked kinase1 overexpression in astrocytoma.Int J Cancer. 2010 Mar 15;126(6):1436-44. doi: 10.1002/ijc.24824. Int J Cancer. 2010. PMID: 19676046
-
Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors.Cancer Res. 2002 Jul 1;62(13):3729-35. Cancer Res. 2002. PMID: 12097282
-
[Angiogenesis and anti-angiogenic strategies for glioblastoma].Bull Cancer. 2005 Apr;92(4):360-72. Bull Cancer. 2005. PMID: 15888393 Review. French.
-
Inhibition of ILK in PTEN-mutant human glioblastomas inhibits PKB/Akt activation, induces apoptosis, and delays tumor growth.Oncogene. 2005 May 19;24(22):3596-605. doi: 10.1038/sj.onc.1208427. Oncogene. 2005. PMID: 15782140
-
[Anti-angiogenic strategies in glioblastoma].Rev Neurol (Paris). 2011 Oct;167(10):662-7. doi: 10.1016/j.neurol.2011.07.008. Epub 2011 Sep 1. Rev Neurol (Paris). 2011. PMID: 21889779 Review. French.
Cited by
-
Antiangiogenic therapy for glioma.J Signal Transduct. 2012;2012:483040. doi: 10.1155/2012/483040. Epub 2012 Jul 8. J Signal Transduct. 2012. PMID: 22830012 Free PMC article.
-
Adipocyte proteome and secretome influence inflammatory and hormone pathways in glioma.Metab Brain Dis. 2019 Feb;34(1):141-152. doi: 10.1007/s11011-018-0327-y. Epub 2018 Oct 9. Metab Brain Dis. 2019. PMID: 30302719
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

