Safety and immunogenicity following administration of a live, attenuated monovalent 2009 H1N1 influenza vaccine to children and adults in two randomized controlled trials
- PMID: 21060780
- PMCID: PMC2966412
- DOI: 10.1371/journal.pone.0013755
Safety and immunogenicity following administration of a live, attenuated monovalent 2009 H1N1 influenza vaccine to children and adults in two randomized controlled trials
Abstract
Background: The safety, tolerability, and immunogenicity of a monovalent intranasal 2009 A/H1N1 live attenuated influenza vaccine (LAIV) were evaluated in children and adults.
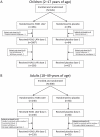
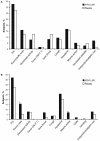
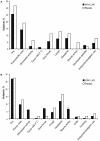
Methods/principal findings: Two randomized, double-blind, placebo-controlled studies were completed in children (2-17 y) and adults (18-49 y). Subjects were assigned 4:1 to receive 2 doses of H1N1 LAIV or placebo 28 days apart. The primary safety endpoint was fever ≥38.3°C during days 1-8 after the first dose; the primary immunogenicity endpoint was the proportion of subjects experiencing a postdose seroresponse. Solicited symptoms and adverse events were recorded for 14 days after each dose and safety data were collected for 180 days post-final dose. In total, 326 children (H1N1 LAIV, n = 261; placebo, n = 65) and 300 adults (H1N1 LAIV, n = 240; placebo, n = 60) were enrolled. After dose 1, fever ≥38.3°C occurred in 4 (1.5%) pediatric vaccine recipients and 1 (1.5%) placebo recipient (rate difference, 0%; 95% CI: -6.4%, 3.1%). No adults experienced fever following dose 1. Seroresponse rates in children (H1N1 LAIV vs. placebo) were 11.1% vs. 6.3% after dose 1 (rate difference, 4.8%; 95% CI: -9.6%, 13.8%) and 32.0% vs. 14.5% after dose 2 (rate difference, 17.5%; 95% CI: 5.5%, 27.1%). Seroresponse rates in adults were 6.1% vs. 0% (rate difference, 6.1%; 95% CI: -5.6%, 12.6%) and 14.9% vs. 5.6% (rate difference, 9.3%; 95% CI: -0.8%, 16.3%) after dose 1 and dose 2, respectively. Solicited symptoms after dose 1 (H1N1 LAIV vs. placebo) occurred in 37.5% vs. 32.3% of children and 41.7% vs. 31.7% of adults. Solicited symptoms occurred less frequently after dose 2 in adults and children. No vaccine-related serious adverse events occurred.
Conclusions/significance: In subjects aged 2 to 49 years, two doses of H1N1 LAIV have a safety and immunogenicity profile similar to other previously studied and efficacious formulations of seasonal trivalent LAIV.
Trial registration: ClinicalTrials.gov NCT00946101, NCT00945893.
Conflict of interest statement
Figures
References
-
- Maassab HF. Biologic and immunologic characteristics of cold-adapted influenza virus. J Immunol. 1969;102:728–732. - PubMed
-
- Jin H, Lu B, Zhou H, Ma C, Zhao J, et al. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology. 2003;306:18–24. - PubMed
-
- Belshe R, Lee MS, Walker RE, Stoddard J, Mendelman PM. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert Rev Vaccines. 2004;3:643–654. - PubMed
-
- Englund JA, Walter EB, Fairchok MP, Monto AS, Neuzil KM. A comparison of 2 influenza vaccine schedules in 6- to 23-month-old children. Pediatrics. 2005;115:1039–1047. - PubMed
-
- Neuzil KM, Jackson LA, Nelson J, Klimov A, Cox N, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5-8-year-old children. J Infect Dis. 2006;194:1032–1039. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

