Nucleocapsid promotes localization of HIV-1 gag to uropods that participate in virological synapses between T cells
- PMID: 21060818
- PMCID: PMC2965768
- DOI: 10.1371/journal.ppat.1001167
Nucleocapsid promotes localization of HIV-1 gag to uropods that participate in virological synapses between T cells
Abstract
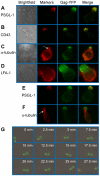
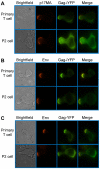
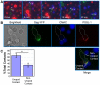
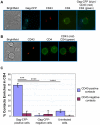
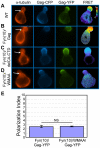
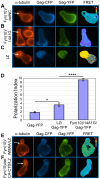
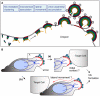
T cells adopt a polarized morphology in lymphoid organs, where cell-to-cell transmission of HIV-1 is likely frequent. However, despite the importance of understanding virus spread in vivo, little is known about the HIV-1 life cycle, particularly its late phase, in polarized T cells. Polarized T cells form two ends, the leading edge at the front and a protrusion called a uropod at the rear. Using multiple uropod markers, we observed that HIV-1 Gag localizes to the uropod in polarized T cells. Infected T cells formed contacts with uninfected target T cells preferentially via HIV-1 Gag-containing uropods compared to leading edges that lack plasma-membrane-associated Gag. Cell contacts enriched in Gag and CD4, which define the virological synapse (VS), are also enriched in uropod markers. These results indicate that Gag-laden uropods participate in the formation and/or structure of the VS, which likely plays a key role in cell-to-cell transmission of HIV-1. Consistent with this notion, a myosin light chain kinase inhibitor, which disrupts uropods, reduced virus particle transfer from infected T cells to target T cells. Mechanistically, we observed that Gag copatches with antibody-crosslinked uropod markers even in non-polarized cells, suggesting an association of Gag with uropod-specific microdomains that carry Gag to uropods. Finally, we determined that localization of Gag to the uropod depends on higher-order clustering driven by its NC domain. Taken together, these results support a model in which NC-dependent Gag accumulation to uropods establishes a preformed platform that later constitutes T-cell-T-cell contacts at which HIV-1 virus transfer occurs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
HIV-1 Gag associates with specific uropod-directed microdomains in a manner dependent on its MA highly basic region.J Virol. 2013 Jun;87(11):6441-54. doi: 10.1128/JVI.00040-13. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536680 Free PMC article.
-
Murine leukemia virus Gag localizes to the uropod of migrating primary lymphocytes.J Virol. 2014 Sep;88(18):10541-55. doi: 10.1128/JVI.01104-14. Epub 2014 Jun 25. J Virol. 2014. PMID: 24965475 Free PMC article.
-
Basic motifs target PSGL-1, CD43, and CD44 to plasma membrane sites where HIV-1 assembles.J Virol. 2015 Jan;89(1):454-67. doi: 10.1128/JVI.02178-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320329 Free PMC article.
-
Roles of Virion-Incorporated CD162 (PSGL-1), CD43, and CD44 in HIV-1 Infection of T Cells.Viruses. 2021 Sep 26;13(10):1935. doi: 10.3390/v13101935. Viruses. 2021. PMID: 34696365 Free PMC article. Review.
-
How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain.Viruses. 2020 Aug 13;12(8):888. doi: 10.3390/v12080888. Viruses. 2020. PMID: 32823718 Free PMC article. Review.
Cited by
-
Viral determinants of polarized assembly for the murine leukemia virus.J Virol. 2011 Aug;85(15):7672-82. doi: 10.1128/JVI.00409-11. Epub 2011 May 18. J Virol. 2011. PMID: 21593177 Free PMC article.
-
Ezrin is a component of the HIV-1 virological presynapse and contributes to the inhibition of cell-cell fusion.J Virol. 2014 Jul;88(13):7645-58. doi: 10.1128/JVI.00550-14. Epub 2014 Apr 23. J Virol. 2014. PMID: 24760896 Free PMC article.
-
Evidence in support of RNA-mediated inhibition of phosphatidylserine-dependent HIV-1 Gag membrane binding in cells.J Virol. 2013 Jun;87(12):7155-9. doi: 10.1128/JVI.00075-13. Epub 2013 Apr 3. J Virol. 2013. PMID: 23552424 Free PMC article.
-
How HIV-1 takes advantage of the cytoskeleton during replication and cell-to-cell transmission.Viruses. 2011 Sep;3(9):1757-76. doi: 10.3390/v3091757. Epub 2011 Sep 15. Viruses. 2011. PMID: 21994805 Free PMC article. Review.
-
Membrane interaction of retroviral Gag proteins.Front Microbiol. 2014 Apr 29;5:187. doi: 10.3389/fmicb.2014.00187. eCollection 2014. Front Microbiol. 2014. PMID: 24808894 Free PMC article. Review.
References
-
- Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, et al. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. - PubMed
-
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. - PubMed
-
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

