An automated phenotype-driven approach (GeneForce) for refining metabolic and regulatory models
- PMID: 21060853
- PMCID: PMC2965739
- DOI: 10.1371/journal.pcbi.1000970
An automated phenotype-driven approach (GeneForce) for refining metabolic and regulatory models
Abstract
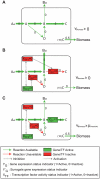
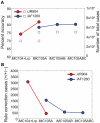
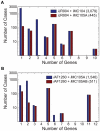
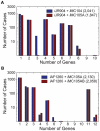
Integrated constraint-based metabolic and regulatory models can accurately predict cellular growth phenotypes arising from genetic and environmental perturbations. Challenges in constructing such models involve the limited availability of information about transcription factor--gene target interactions and computational methods to quickly refine models based on additional datasets. In this study, we developed an algorithm, GeneForce, to identify incorrect regulatory rules and gene-protein-reaction associations in integrated metabolic and regulatory models. We applied the algorithm to refine integrated models of Escherichia coli and Salmonella typhimurium, and experimentally validated some of the algorithm's suggested refinements. The adjusted E. coli model showed improved accuracy (∼80.0%) for predicting growth phenotypes for 50,557 cases (knockout mutants tested for growth in different environmental conditions). In addition to identifying needed model corrections, the algorithm was used to identify native E. coli genes that, if over-expressed, would allow E. coli to grow in new environments. We envision that this approach will enable the rapid development and assessment of genome-scale metabolic and regulatory network models for less characterized organisms, as such models can be constructed from genome annotations and cis-regulatory network predictions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Herrgard MJ, Covert MW, Palsson BO. Reconstruction of microbial transcriptional regulatory networks. Curr Opin Biotechnol. 2004;15:70–77. - PubMed
-
- Covert MW, Knight EM, Reed JL, Herrgard MJ, Palsson BO. Integrating high-throughput and computational data elucidates bacterial networks. Nature. 2004;429:92–96. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

