Toxic but drank: gustatory aversive compounds induce post-ingestional malaise in harnessed honeybees
- PMID: 21060877
- PMCID: PMC2965165
- DOI: 10.1371/journal.pone.0015000
Toxic but drank: gustatory aversive compounds induce post-ingestional malaise in harnessed honeybees
Abstract
Background: Deterrent substances produced by plants are relevant due to their potential toxicity. The fact that most of these substances have an unpalatable taste for humans and other mammals contrasts with the fact that honeybees do not reject them in the range of concentrations in which these compounds are present in flower nectars. Here we asked whether honeybees detect and ingest deterrent substances and whether these substances are really toxic to them.
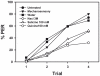
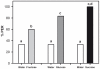
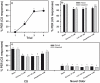
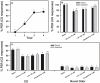
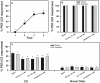
Results: We show that pairing aversive substances with an odor retards learning of this odor when it is subsequently paired with sucrose. Harnessed honeybees in the laboratory ingest without reluctance a considerable volume (20 µl) of various aversive substances, even if some of them induce significant post-ingestional mortality. These substances do not seem, therefore, to be unpalatable to harnessed bees but induce a malaise-like state that in some cases results in death. Consistently with this finding, bees learning that one odor is associated with sugar, and experiencing in a subsequent phase that the sugar was paired with 20 µl of an aversive substance (devaluation phase), respond less than control bees to the odor and the sugar. Such stimulus devaluation can be accounted for by the malaise-like state induced by the aversive substances.
Conclusion: Our results indicate that substances that taste bitter to humans as well as concentrated saline solutions base their aversive effect on the physiological consequences that their ingestion generates in harnessed bees rather than on an unpalatable taste. This conclusion is only valid for harnessed bees in the laboratory as freely-moving bees might react differently to aversive compounds could actively reject aversive substances. Our results open a new possibility to study conditioned taste aversion based on post-ingestional malaise and thus broaden the spectrum of aversive learning protocols available in honeybees.
Conflict of interest statement
Figures

References
-
- Scott K. The sweet and the bitter of mammalian taste. Curr Opin Neurobiol. 2004;14:423–7. - PubMed
-
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, et al. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. - PubMed
-
- Wink M. Plant breeding importance of secondary metabolites for protection against pathogens and herbivores. Theor Appl Genet. 1988;75:225–233.
-
- Wittstock U, Gershenzon J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr Opin Plant Biol. 2002;5:300–307. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

