Expression and distribution of immunoglobulin G and its receptors in an immune privileged site: the eye
- PMID: 21061041
- PMCID: PMC11114928
- DOI: 10.1007/s00018-010-0572-7
Expression and distribution of immunoglobulin G and its receptors in an immune privileged site: the eye
Abstract
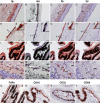
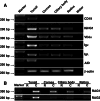
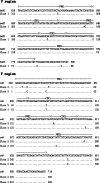
It has recently been demonstrated that not only mature B lymphocytes, but also non-lymphoid cells, including cancer cells and neurons, express IgG. In the eye, an important immune privileged site, the presence of IgG has been ascribed to IgG entering the eye through breaches of the blood–ocular barrier. Here we demonstrate that the eye itself can produce IgG intrinsically. Applying immunohistochemistry, in situ hybridization, and RT-PCR, several intraocular structures were found to express proteins and mRNA transcripts of IgG heavy chains, light chains, V(D)J rearrangements, and enzymes required for V(D)J recombination. IgG receptors were also detected in the intraocular epithelium and endothelium. The extensive distribution of IgG and its receptors in intraocular structures indicates that locally produced IgG could play a significant role in maintaining the ocular microenvironment and protection of the eyes, and it might also be involved in the pathogenesis of age-related macular degeneration and some inflammatory diseases.
Figures



Similar articles
-
Immunoglobulin G expression and its colocalization with complement proteins in papillary thyroid cancer.Mod Pathol. 2012 Jan;25(1):36-45. doi: 10.1038/modpathol.2011.139. Epub 2011 Sep 9. Mod Pathol. 2012. PMID: 21909078
-
Expression of immunoglobulin G in esophageal squamous cell carcinomas and its association with tumor grade and Ki67.Hum Pathol. 2012 Mar;43(3):423-34. doi: 10.1016/j.humpath.2011.05.020. Epub 2011 Aug 19. Hum Pathol. 2012. PMID: 21855109
-
Immunoglobulin G expression in carcinomas and cancer cell lines.FASEB J. 2007 Sep;21(11):2931-8. doi: 10.1096/fj.07-8073com. Epub 2007 May 2. FASEB J. 2007. PMID: 17475920
-
Recombination centres and the orchestration of V(D)J recombination.Nat Rev Immunol. 2011 Apr;11(4):251-63. doi: 10.1038/nri2941. Epub 2011 Mar 11. Nat Rev Immunol. 2011. PMID: 21394103 Review.
-
RAGs' eye view of the immunoglobulin heavy chain gene locus.Semin Immunol. 2010 Dec;22(6):337-45. doi: 10.1016/j.smim.2010.08.003. Semin Immunol. 2010. PMID: 20864355 Review.
Cited by
-
Exploring the Origin and Physiological Significance of DNA Double Strand Breaks in the Developing Neuroretina.Int J Mol Sci. 2022 Jun 9;23(12):6449. doi: 10.3390/ijms23126449. Int J Mol Sci. 2022. PMID: 35742893 Free PMC article. Review.
-
Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells.Oncogene. 2017 Aug 24;36(34):4887-4900. doi: 10.1038/onc.2017.72. Epub 2017 Apr 24. Oncogene. 2017. PMID: 28436947 Free PMC article.
-
Impact of Autoantibodies against Glycolytic Enzymes on Pathogenicity of Autoimmune Retinopathy and Other Autoimmune Disorders.Front Immunol. 2017 Apr 28;8:505. doi: 10.3389/fimmu.2017.00505. eCollection 2017. Front Immunol. 2017. PMID: 28503176 Free PMC article. Review.
-
Naturally Acquired Antibodies against Plasmodium falciparum: Friend or Foe?Pathogens. 2021 Jul 2;10(7):832. doi: 10.3390/pathogens10070832. Pathogens. 2021. PMID: 34357982 Free PMC article. Review.
-
Expression of immunoglobulin G in human proximal tubular epithelial cells.Mol Med Rep. 2021 May;23(5):327. doi: 10.3892/mmr.2021.11966. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760139 Free PMC article.
References
-
- Lee HO, Herndon JM, Barreiro R, Griffith TS, Ferguson TA. TRAIL: a mechanism of tumor surveillance in an immune privileged site. J Immunol. 2002;169:4739–4744. - PubMed
-
- Allansmith MR, Whitney CR, McClellan BH, Newman LP. Immunoglobulins in the human eye. Location, type, and amount. Arch Ophthalmol. 1973;89:36–45. - PubMed
-
- Waldrep JC, Schulte JR. Characterization of human IgG subclasses within intraocular compartments. Reg Immunol. 1989;2:22–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

