B7-H1 expression on non-B and non-T cells promotes distinct effects on T- and B-cell responses in autoimmune arthritis
- PMID: 21061440
- PMCID: PMC3638795
- DOI: 10.1002/eji.201040690
B7-H1 expression on non-B and non-T cells promotes distinct effects on T- and B-cell responses in autoimmune arthritis
Abstract
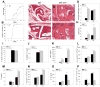
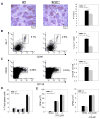
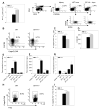
The immune system has developed several regulatory mechanisms to maintain homeostasis of adaptive immune responses. T-cell programmed death (PD)-1 recognition of B7-H1 (PD-L1) expressed on APC and non-lymphoid tissue regulates T-cell activation. We show that B7-H1(-/-) mice exhibit exacerbated proteoglycan (PG)-induced arthritis and increased Th-1 CD4(+) T-cell responses. Unexpectedly, the PG-specific antibody response in B7-H1(-/-) mice was diminished. A reduction in the number of peanut agglutinin(+) GC coincided with a decrease in CD19(+) GL-7(+) CD95(+) GC B cells that was a result of increased caspase-induced apoptosis. The percent of CD38(+) CD138(+) emerging plasma cells was decreased. B7-H1(-/-) mice exhibited an increased frequency of CD4(+) PD-1(hi) CXCR5(hi) ICOS(hi) CD62L(lo) T follicular helper cells that displayed a hyperactive phenotype with increased expression of mRNA transcripts for Bcl6, IL-21, and the apoptosis-inducer molecule FasL. In cell transfer of B7-H1(-/-) cells into SCID mice, non-B and non-T cells were sufficient to normalize the antibody response, T-cell hyperactivity, and the development of PG-induced arthritis. These findings indicate that B7-H1 on non-B and non-T cells signals through PD-1 on T effector cells to prevent excessive activation and reduce autoimmune arthritis. Furthermore, these findings demonstrate a novel role for B7-H1 expression in promoting B-cell survival by regulating the activation of T follicular helper cell.
Copyright © 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE.Eur J Immunol. 2008 Oct;38(10):2706-17. doi: 10.1002/eji.200838137. Eur J Immunol. 2008. PMID: 18825752 Free PMC article.
-
Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy.Blood. 2007 Jul 1;110(1):180-5. doi: 10.1182/blood-2006-11-060087. Epub 2007 Feb 8. Blood. 2007. PMID: 17289811 Free PMC article.
-
Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo.J Immunol. 2005 Jun 1;174(11):6648-56. doi: 10.4049/jimmunol.174.11.6648. J Immunol. 2005. PMID: 15905503
-
Negative regulation of T-cell function by PD-1.Crit Rev Immunol. 2004;24(4):229-37. doi: 10.1615/critrevimmunol.v24.i4.10. Crit Rev Immunol. 2004. PMID: 15588223 Review.
-
Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy.Cancer Immunol Immunother. 2005 Apr;54(4):307-14. doi: 10.1007/s00262-004-0593-x. Epub 2004 Dec 15. Cancer Immunol Immunother. 2005. PMID: 15599732 Free PMC article. Review.
Cited by
-
Immunotherapy in Breast Cancer: the Emerging Role of PD-1 and PD-L1.Curr Oncol Rep. 2017 Aug 10;19(10):64. doi: 10.1007/s11912-017-0627-0. Curr Oncol Rep. 2017. PMID: 28799073 Review.
-
CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation.J Immunol. 2012 May 1;188(9):4217-25. doi: 10.4049/jimmunol.1102885. Epub 2012 Mar 26. J Immunol. 2012. PMID: 22450810 Free PMC article.
-
The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood.Nat Immunol. 2013 Feb;14(2):152-61. doi: 10.1038/ni.2496. Epub 2012 Dec 16. Nat Immunol. 2013. PMID: 23242415 Free PMC article.
-
Bridging the Gap: Connecting the Mechanisms of Immune-Related Adverse Events and Autoimmunity Through PD-1.Front Cell Dev Biol. 2022 Jan 3;9:790386. doi: 10.3389/fcell.2021.790386. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35047501 Free PMC article. Review.
-
Cognate interaction with iNKT cells expands IL-10-producing B regulatory cells.Proc Natl Acad Sci U S A. 2015 Oct 6;112(40):12474-9. doi: 10.1073/pnas.1504790112. Epub 2015 Sep 21. Proc Natl Acad Sci U S A. 2015. PMID: 26392556 Free PMC article.
References
-
- Firestein GS. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2005;11:S39–44. - PubMed
-
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. - PubMed
-
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. - PubMed
-
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

