Limiting an antimicrobial peptide to the lipid-water interface enhances its bacterial membrane selectivity: a case study of MSI-367
- PMID: 21062093
- PMCID: PMC3006059
- DOI: 10.1021/bi101394r
Limiting an antimicrobial peptide to the lipid-water interface enhances its bacterial membrane selectivity: a case study of MSI-367
Abstract
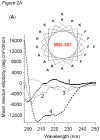
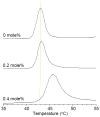
In a minimalist design approach, a synthetic peptide MSI-367 [(KFAKKFA)(3)-NH(2)] was designed and synthesized with the objective of generating cell-selective nonlytic peptides, which have a significant bearing on cell targeting. The peptide exhibited potent activity against both bacteria and fungi, but no toxicity to human cells at micromolar concentrations. Bacterial versus human cell membrane selectivity of the peptide was determined via membrane permeabilization assays. Circular dichroism investigations revealed the intrinsic helix propensity of the peptide, β-turn structure in aqueous buffer and extended and turn conformations upon binding to lipid vesicles. Differential scanning calorimetry experiments with 1,2-dipalmitoleoyl-sn-glycero-3-phosphatidylethanolamine bilayers indicated the induction of positive curvature strain and repression of the fluid lamellar to inverted hexagonal phase transition by MSI-367. Results of isothermal titration calorimetry (ITC) experiments suggested the possibility of formation of specific lipid-peptide complexes leading to aggregation. (2)H nuclear magnetic resonance (NMR) of deuterated 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC) multilamellar vesicles confirmed the limited effect of the membrane-embedded peptide at the lipid-water interface. (31)P NMR data indicated changes in the lipid headgroup orientation of POPC, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylglycerol, and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylethanolamine lipid bilayers upon peptide binding. Membrane-embedded and membrane-inserted states of the peptide were observed via sum frequency generation vibrational spectroscopy. Circular dichroism, ITC, and (31)P NMR data for Escherichia coli lipids agree with the hypothesis that strong electrostatic lipid-peptide interactions embrace the peptide at the lipid-water interface and provide the basis for bacterial cell selectivity.
Figures

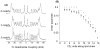



Similar articles
-
Antimicrobial activity and membrane selective interactions of a synthetic lipopeptide MSI-843.Biochim Biophys Acta. 2005 Jun 1;1711(1):49-58. doi: 10.1016/j.bbamem.2005.02.010. Epub 2005 Mar 7. Biochim Biophys Acta. 2005. PMID: 15904663 Free PMC article.
-
The helical propensity of KLA amphipathic peptides enhances their binding to gel-state lipid membranes.Biophys Chem. 2013 Oct-Nov;180-181:10-21. doi: 10.1016/j.bpc.2013.05.003. Epub 2013 May 24. Biophys Chem. 2013. PMID: 23792704
-
MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain.Biophys J. 2003 May;84(5):3052-60. doi: 10.1016/S0006-3495(03)70031-9. Biophys J. 2003. PMID: 12719236 Free PMC article.
-
An Antimicrobial Peptide-Mimetic Methacrylate Random Copolymer Induces Domain Formation in a Model Bacterial Membrane.J Membr Biol. 2022 Oct;255(4-5):513-521. doi: 10.1007/s00232-022-00220-6. Epub 2022 Feb 18. J Membr Biol. 2022. PMID: 35182193 Review.
-
Solution NMR studies of cell-penetrating peptides in model membrane systems.Adv Drug Deliv Rev. 2013 Jul;65(8):1002-11. doi: 10.1016/j.addr.2012.10.011. Epub 2012 Nov 6. Adv Drug Deliv Rev. 2013. PMID: 23137785 Review.
Cited by
-
Impact of membrane curvature on amyloid aggregation.Biochim Biophys Acta Biomembr. 2018 Sep;1860(9):1741-1764. doi: 10.1016/j.bbamem.2018.04.012. Epub 2018 Apr 28. Biochim Biophys Acta Biomembr. 2018. PMID: 29709613 Free PMC article. Review.
-
Shorter Antibacterial Peptide Having High Selectivity for E. coli Membranes and Low Potential for Inducing Resistance.Microorganisms. 2020 Jun 8;8(6):867. doi: 10.3390/microorganisms8060867. Microorganisms. 2020. PMID: 32521823 Free PMC article.
-
How to Combat Gram-Negative Bacteria Using Antimicrobial Peptides: A Challenge or an Unattainable Goal?Antibiotics (Basel). 2021 Dec 7;10(12):1499. doi: 10.3390/antibiotics10121499. Antibiotics (Basel). 2021. PMID: 34943713 Free PMC article. Review.
-
Protein-phospholipid interactions in nonclassical protein secretion: problem and methods of study.Int J Mol Sci. 2013 Feb 8;14(2):3734-72. doi: 10.3390/ijms14023734. Int J Mol Sci. 2013. PMID: 23396106 Free PMC article.
-
Exploring the role of unnatural amino acids in antimicrobial peptides.Sci Rep. 2018 Jun 11;8(1):8888. doi: 10.1038/s41598-018-27231-5. Sci Rep. 2018. PMID: 29892005 Free PMC article.
References
-
- Giuliani A, Pirri G, Nicoletto SF. Antimicrobial peptides: an overview of a promising class of therapeutics. Centr Eur J Biol. 2007;2:1–33.
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. - PubMed
-
- Shai Y, Makovitzky A, Avrahami D. Host defense peptides and lipopeptides: Modes of action and potential candidates for the treatment of bacterial and fungal infections. Curr Protein Pept Sci. 2006;7:479–486. - PubMed
-
- Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnol. 2006;24:1551–1557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

