Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications
- PMID: 21062101
- PMCID: PMC3040277
- DOI: 10.1021/ar1000633
Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications
Abstract
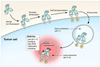
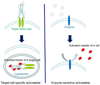
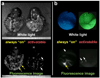
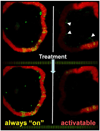
Conventional imaging methods, such as angiography, computed tomography (CT), magnetic resonance imaging (MRI), and radionuclide imaging, rely on contrast agents (iodine, gadolinium, and radioisotopes, for example) that are "always on." Although these indicators have proven clinically useful, their sensitivity is lacking because of inadequate target-to-background signal ratio. A unique aspect of optical imaging is that fluorescence probes can be designed to be activatable, that is, only "turned on" under certain conditions. These probes are engineered to emit signal only after binding a target tissue; this design greatly increases sensitivity and specificity in the detection of disease. Current research focuses on two basic types of activatable fluorescence probes. The first developed were conventional enzymatically activatable probes. These fluorescent molecules exist in the quenched state until activated by enzymatic cleavage, which occurs mostly outside of the cells. However, more recently, researchers have begun designing target-cell-specific activatable probes. These fluorophores exist in the quenched state until activated within targeted cells by endolysosomal processing, which results when the probe binds specific receptors on the cell surface and is subsequently internalized. In this Account, we present a review of the rational design and in vivo applications of target-cell-specific activatable probes. In engineering these probes, researchers have asserted control over a variety of factors, including photochemistry, pharmacological profile, and biological properties. Their progress has recently allowed the rational design and synthesis of target-cell-specific activatable fluorescence imaging probes, which can be conjugated to a wide variety of targeting molecules. Several different photochemical mechanisms have been utilized, each of which offers a unique capability for probe design. These include self-quenching, homo- and hetero-fluorescence resonance energy transfer (FRET), H-dimer formation, and photon-induced electron transfer (PeT). In addition, the repertoire is further expanded by the option for reversibility or irreversibility of the signal emitted through these mechanisms. Given the wide range of photochemical mechanisms and properties, target-cell-specific activatable probes have considerable flexibility and can be adapted to specific diagnostic needs. A multitude of cell surface molecules, such as overexpressed growth factor receptors, are directly related to carcinogenesis and thus provide numerous targets highly specific for cancer. This discussion of the chemical, pharmacological, and biological basis of target-cell-specific activatable imaging probes, and methods for successfully designing them, underscores the systematic, rational basis for further developing in vivo cancer imaging.
Figures



References
-
- Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. - PubMed
-
- Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18:321–325. - PubMed
-
- Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

