Intraperitoneal therapy for peritoneal cancer
- PMID: 21062160
- PMCID: PMC3076138
- DOI: 10.2217/fon.10.100
Intraperitoneal therapy for peritoneal cancer
Abstract
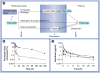
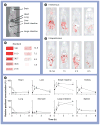
Cancers originating from organs in the peritoneal cavity (e.g., ovarian, pancreatic, colorectal, gastric and liver) account for approximately 250,000 new cancer cases annually in the USA. Peritoneal metastases are common owing to locoregional spread and distant metastases of extraperitoneal cancers. A logical treatment is intraperitoneal therapy, as multiple studies have shown significant targeting advantage for this treatment, including significant survival benefits in stage III, surgically debulked ovarian cancer patients. However, the clinical use of intraperitoneal therapy has been limited, in part, by toxicity, owing to the use of indwelling catheters or high drug exposure, by inadequate drug penetration into bulky tumors (>1 cm) and by the lack of products specifically designed and approved for intraperitoneal treatments. This article provides an overview on the background of peritoneal metastasis, clinical research on intraperitoneal therapy, the pharmacokinetic basis of drug delivery in intraperitoneal therapy and our development of drug-loaded tumor-penetrating microparticles.
Figures




Similar articles
-
Pharmacokinetic and pharmacodynamic optimization of intraperitoneal chemotherapy.Life Sci. 1996;58(7):535-43. doi: 10.1016/0024-3205(95)02200-7. Life Sci. 1996. PMID: 8632706 Review.
-
[Importance and problems of intraperitoneal chemotherapy].Wien Med Wochenschr. 1990 Apr 30;140(8):199-202. Wien Med Wochenschr. 1990. PMID: 2194375 Review. German.
-
Experimental and pharmacokinetic studies in intraperitoneal chemotherapy: from laboratory bench to bedside.Recent Results Cancer Res. 2007;169:53-73. doi: 10.1007/978-3-540-30760-0_5. Recent Results Cancer Res. 2007. PMID: 17506249 No abstract available.
-
Intraperitoneal chemotherapy for peritoneal metastases: an expert opinion.Expert Opin Drug Deliv. 2020 Apr;17(4):511-522. doi: 10.1080/17425247.2020.1736551. Epub 2020 Mar 18. Expert Opin Drug Deliv. 2020. PMID: 32142389 Review.
-
Intraperitoneal delivery of chemotherapeutic agents for the treatment of peritoneal metastases: current challenges and how to overcome them.Expert Opin Drug Deliv. 2019 Dec;16(12):1393-1401. doi: 10.1080/17425247.2019.1693997. Epub 2019 Nov 26. Expert Opin Drug Deliv. 2019. PMID: 31725340 Review.
Cited by
-
Interactions between Cisplatin and Quercetin at Physiological and Hyperthermic Conditions on Cancer Cells In Vitro and In Vivo.Molecules. 2020 Jul 17;25(14):3271. doi: 10.3390/molecules25143271. Molecules. 2020. PMID: 32709143 Free PMC article.
-
Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis.Sci Rep. 2016 Aug 4;6:30696. doi: 10.1038/srep30696. Sci Rep. 2016. PMID: 27488951 Free PMC article.
-
Polycation fluorination improves intraperitoneal siRNA delivery in metastatic pancreatic cancer.J Control Release. 2021 May 10;333:139-150. doi: 10.1016/j.jconrel.2021.03.028. Epub 2021 Mar 25. J Control Release. 2021. PMID: 33774121 Free PMC article.
-
Aggregation-induced emission luminogens for image-guided surgery in non-human primates.Nat Commun. 2021 Nov 10;12(1):6485. doi: 10.1038/s41467-021-26417-2. Nat Commun. 2021. PMID: 34759280 Free PMC article.
-
Intraperitoneal siRNA Nanoparticles for Augmentation of Gemcitabine Efficacy in the Treatment of Pancreatic Cancer.Mol Pharm. 2021 Dec 6;18(12):4448-4458. doi: 10.1021/acs.molpharmaceut.1c00653. Epub 2021 Oct 26. Mol Pharm. 2021. PMID: 34699242 Free PMC article.
References
Bibliography
-
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–363. - PubMed
-
- Au JL, Jang SH, Wientjes MG. Clinical aspects of drug delivery to tumors. J Controlled Release. 2002;78(1–3):81–95. - PubMed
-
- Jain RK. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6(4):559–593. - PubMed
-
- Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res. 2003;20(9):1337–1350. - PubMed
Websites
-
- National Cancer Institute. NCI issues clinical announcement for preferred method of treatment for advanced ovarian cancer. 2006. www.cancer.gov/newscenter/pressreleases/IPchemotherapyrelease.
-
- European Medicines Agency. Removab: summary of product characteristics. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information....
-
- US FDA access data on leuprolide acetate. www.accessdata.fda.gov/drugsatfda_docs/label/2009/019943s029,020011s036l....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
