Genetic regulation of human brain development: lessons from Mendelian diseases
- PMID: 21062301
- PMCID: PMC4915827
- DOI: 10.1111/j.1749-6632.2010.05819.x
Genetic regulation of human brain development: lessons from Mendelian diseases
Abstract
One of the fundamental goals in human genetics is to link gene function to phenotype, yet the function of the majority of the genes in the human body is still poorly understood. This is especially true for the developing human brain. The study of human phenotypes that result from inherited, mutated alleles is the most direct evidence for the requirement of a gene in human physiology. Thus, the study of Mendelian central nervous system (CNS) diseases can be an extremely powerful approach to elucidate such phenotypic/genotypic links and to increase our understanding of the key components required for development of the human brain. In this review, we highlight examples of how the study of inherited neurodevelopmental disorders contributes to our knowledge of both the "normal" and diseased human brain, as well as elaborate on the future of this type of research. Mendelian disease research has been, and will continue to be, key to understanding the molecular mechanisms that underlie human brain function, and will ultimately form a basis for the design of intelligent, mechanism-specific treatments for nervous system disorders.
© 2010 New York Academy of Sciences.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

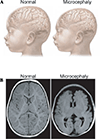

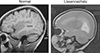
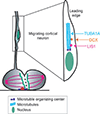
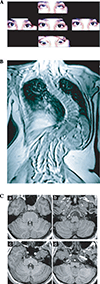

References
-
- Drachman DA. Do we have brain to spare? Neurology. 2005;64:2004–2005. - PubMed
-
- International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. - PubMed
-
- Peltonen L, et al. Lessons from studying monogenic disease for common disease. Hum. Mol. Genet. 2006;15(Spec. No. 1):R67–R74. - PubMed
-
- Engel J, Pedley TA. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven; 1998.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

