Cardiac resynchronization therapy guided by cardiovascular magnetic resonance
- PMID: 21062491
- PMCID: PMC2994940
- DOI: 10.1186/1532-429X-12-64
Cardiac resynchronization therapy guided by cardiovascular magnetic resonance
Abstract
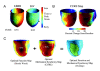
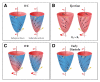
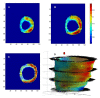
Cardiac resynchronization therapy (CRT) is an established treatment for patients with symptomatic heart failure, severely impaired left ventricular (LV) systolic dysfunction and a wide (> 120 ms) complex. As with any other treatment, the response to CRT is variable. The degree of pre-implant mechanical dyssynchrony, scar burden and scar localization to the vicinity of the LV pacing stimulus are known to influence response and outcome. In addition to its recognized role in the assessment of LV structure and function as well as myocardial scar, cardiovascular magnetic resonance (CMR) can be used to quantify global and regional LV dyssynchrony. This review focuses on the role of CMR in the assessment of patients undergoing CRT, with emphasis on risk stratification and LV lead deployment.
Figures












References
-
- Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, Klein H, Karmer A, Ding J, Salo R. et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation. 1999;99:2993–3001. - PubMed
-
- Cazeau S, Leclercq C, Lavergene T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M. et al. Effects of multisite biventricular pacing in patients with heart failure and interventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. - DOI - PubMed
-
- Cleland JGF, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. for the Cardiac Resynchronization-Heart Failure (CARE-HF) study investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Eng J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

