The FtsH protease heterocomplex in Arabidopsis: dispensability of type-B protease activity for proper chloroplast development
- PMID: 21062893
- PMCID: PMC3015133
- DOI: 10.1105/tpc.110.079202
The FtsH protease heterocomplex in Arabidopsis: dispensability of type-B protease activity for proper chloroplast development
Abstract
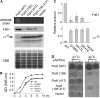
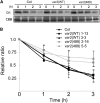
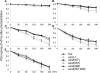
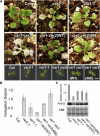
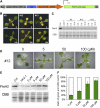
FtsH is an ATP-dependent metalloprotease present as a hexameric heterocomplex in thylakoid membranes. Encoded in the Arabidopsis thaliana YELLOW VARIEGATED2 (VAR2) locus, FtsH2 is one isoform among major Type A (FtsH1/5) and Type B (FtsH2/8) isomers. Mutants lacking FtsH2 (var2) and FtsH5 (var1) are characterized by a typical leaf-variegated phenotype. The functional importance of the catalytic center (comprised by the zinc binding domain) in FtsH2 was assessed in this study by generating transgenic plants that ectopically expressed FtsH2(488), a proteolytically inactive version of FtsH2. The resulting amino acid substitution inhibited FtsH protease activity in vivo when introduced into Escherichia coli FtsH. By contrast, expression of FtsH2(488) rescued not only leaf variegation in var2 but also seedling lethality in var2 ftsh8, suggesting that the protease activity of Type B isomers is completely dispensable, which implies that the chloroplastic FtsH complex has protease sites in excess and that they act redundantly rather than coordinately. However, expression of FtsH2(488) did not fully rescue leaf variegation in var1 var2 because the overall FtsH levels were reduced under this background. Applying an inducible promoter to our complementation analysis revealed that rescue of leaf variegation indeed depends on the overall amount of FtsH. Our results elucidate protein activity and its amount as important factors for the function of FtsH heterocomplexes that are composed of multiple isoforms in the thylakoid membrane.
Figures


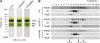

Similar articles
-
Coordinated regulation and complex formation of yellow variegated1 and yellow variegated2, chloroplastic FtsH metalloproteases involved in the repair cycle of photosystem II in Arabidopsis thylakoid membranes.Plant Cell. 2003 Dec;15(12):2843-55. doi: 10.1105/tpc.017319. Epub 2003 Nov 20. Plant Cell. 2003. PMID: 14630971 Free PMC article.
-
Allelic characterization of the leaf-variegated mutation var2 identifies the conserved amino acid residues of FtsH that are important for ATP hydrolysis and proteolysis.Plant Mol Biol. 2004 Nov;56(5):705-16. doi: 10.1007/s11103-004-4561-9. Epub 2005 Mar 24. Plant Mol Biol. 2004. PMID: 15803409
-
The balance between protein synthesis and degradation in chloroplasts determines leaf variegation in Arabidopsis yellow variegated mutants.Plant Cell. 2007 Apr;19(4):1313-28. doi: 10.1105/tpc.106.049270. Epub 2007 Apr 6. Plant Cell. 2007. PMID: 17416734 Free PMC article.
-
Arabidopsis chloroplast FtsH, var2 and suppressors of var2 leaf variegation: a review.J Integr Plant Biol. 2010 Aug;52(8):750-61. doi: 10.1111/j.1744-7909.2010.00980.x. J Integr Plant Biol. 2010. PMID: 20666930 Review.
-
FtsH Protease in the Thylakoid Membrane: Physiological Functions and the Regulation of Protease Activity.Front Plant Sci. 2018 Jun 20;9:855. doi: 10.3389/fpls.2018.00855. eCollection 2018. Front Plant Sci. 2018. PMID: 29973948 Free PMC article. Review.
Cited by
-
The Zinc-Finger Thylakoid-Membrane Protein FIP Is Involved With Abiotic Stress Response in Arabidopsis thaliana.Front Plant Sci. 2018 Apr 18;9:504. doi: 10.3389/fpls.2018.00504. eCollection 2018. Front Plant Sci. 2018. PMID: 29720990 Free PMC article.
-
Fixing PSII: Membrane fluidity facilitates FtsH functions.Plant Cell. 2024 Dec 23;37(1):koaf003. doi: 10.1093/plcell/koaf003. Plant Cell. 2024. PMID: 39761687 Free PMC article. No abstract available.
-
Cross-talk between calcium signalling and protein phosphorylation at the thylakoid.J Exp Bot. 2012 Feb;63(4):1725-33. doi: 10.1093/jxb/err403. Epub 2011 Dec 22. J Exp Bot. 2012. PMID: 22197893 Free PMC article.
-
A critical role of PvFtsH2 in the degradation of photodamaged D1 protein in common bean.Hortic Res. 2021 Jun 1;8(1):126. doi: 10.1038/s41438-021-00554-7. Hortic Res. 2021. PMID: 34059658 Free PMC article.
-
Thylakoid-Bound FtsH Proteins Facilitate Proper Biosynthesis of Photosystem I.Plant Physiol. 2016 Jun;171(2):1333-43. doi: 10.1104/pp.16.00200. Epub 2016 May 2. Plant Physiol. 2016. PMID: 27208291 Free PMC article.
References
-
- Adam Z., Rudella A., van Wijk K.J. (2006). Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr. Opin. Plant Biol. 9: 234–240 - PubMed
-
- Aluru M.R., Yu F., Fu A., Rodermel S. (2006). Arabidopsis variegation mutants: New insights into chloroplast biogenesis. J. Exp. Bot. 57: 1871–1881 - PubMed
-
- Bailey S., Thompson E., Nixon P.J., Horton P., Mullineaux C.W., Robinson C., Mann N.H. (2002). A critical role for the Var2 FtsH homologue of Arabidopsis thaliana in the photosystem II repair cycle in vivo. J. Biol. Chem. 277: 2006–2011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

