Agonist-activated Ca2+ influx occurs at stable plasma membrane and endoplasmic reticulum junctions
- PMID: 21062895
- PMCID: PMC2987444
- DOI: 10.1242/jcs.068387
Agonist-activated Ca2+ influx occurs at stable plasma membrane and endoplasmic reticulum junctions
Abstract
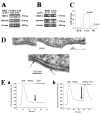
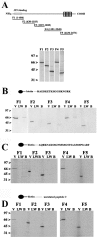
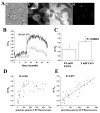
Junctate is a 33 kDa integral protein of sarco(endo)plasmic reticulum membranes that forms a macromolecular complex with inositol 1,4,5-trisphosphate [Ins(1,4,5)P(3)] receptors and TRPC3 channels. TIRF microscopy shows that junctate enhances the number of fluorescent puncta on the plasma membrane. The size and distribution of these puncta are not affected by the addition of agonists that mobilize Ca(2+) from Ins(1,4,5)P(3)-sensitive stores. Puncta are associated with a significantly larger number of peripheral junctions between endoplasmic reticulum and plasma membrane, which are further enhanced upon stable co-expression of junctate and TRPC3. The gap between the membranes of peripheral junctions is bridged by regularly spaced electron-dense structures of 10 nm. Ins(1,4,5)P(3) inhibits the interaction of the cytoplasmic N-terminus of junctate with the ligand-binding domain of the Ins(1,4,5)P(3) receptor. Furthermore, Ca(2+) influx evoked by activation of Ins(1,4,5)P(3) receptors is increased where puncta are located. We conclude that stable peripheral junctions between the plasma membrane and endoplasmic reticulum are the anatomical sites of agonist-activated Ca(2+) entry.
Figures



Similar articles
-
Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion.J Cell Biol. 2004 Aug 16;166(4):537-48. doi: 10.1083/jcb.200404079. Epub 2004 Aug 9. J Cell Biol. 2004. PMID: 15302852 Free PMC article.
-
Junctate, an inositol 1,4,5-triphosphate receptor associated protein, is present in rodent sperm and binds TRPC2 and TRPC5 but not TRPC1 channels.Dev Biol. 2005 Oct 1;286(1):326-37. doi: 10.1016/j.ydbio.2005.08.006. Dev Biol. 2005. PMID: 16153633
-
Junctate boosts phagocytosis by recruiting endoplasmic reticulum Ca2+ stores near phagosomes.J Cell Sci. 2015 Nov 15;128(22):4074-82. doi: 10.1242/jcs.172510. Epub 2015 Oct 7. J Cell Sci. 2015. PMID: 26446257
-
New Aspects of the Contribution of ER to SOCE Regulation: TRPC Proteins as a Link Between Plasma Membrane Ion Transport and Intracellular Ca2+ Stores.Adv Exp Med Biol. 2017;993:239-255. doi: 10.1007/978-3-319-57732-6_13. Adv Exp Med Biol. 2017. PMID: 28900918 Review.
-
Protein-protein interaction and functionTRPC channels.Pflugers Arch. 2005 Oct;451(1):116-24. doi: 10.1007/s00424-005-1442-2. Epub 2005 Jul 26. Pflugers Arch. 2005. PMID: 16044307 Review.
Cited by
-
Pathophysiological significance and modulation of the transient receptor potential canonical 3 ion channel.Med Res Rev. 2024 Nov;44(6):2510-2544. doi: 10.1002/med.22048. Epub 2024 May 7. Med Res Rev. 2024. PMID: 38715347 Review.
-
A FAIR, open-source virtual reality platform for dendritic spine analysis.Patterns (N Y). 2024 Aug 12;5(9):101041. doi: 10.1016/j.patter.2024.101041. eCollection 2024 Sep 13. Patterns (N Y). 2024. PMID: 39568639 Free PMC article.
-
Molecular mechanisms of STIM/Orai communication.Am J Physiol Cell Physiol. 2016 Apr 15;310(8):C643-62. doi: 10.1152/ajpcell.00007.2016. Epub 2016 Jan 28. Am J Physiol Cell Physiol. 2016. PMID: 26825122 Free PMC article. Review.
-
Orai1, STIM1, and their associating partners.J Physiol. 2012 Sep 1;590(17):4169-77. doi: 10.1113/jphysiol.2012.231522. Epub 2012 May 14. J Physiol. 2012. PMID: 22586216 Free PMC article. Review.
-
Identification of ER/SR resident proteins as biomarkers for ER/SR calcium depletion in skeletal muscle cells.Orphanet J Rare Dis. 2022 Jun 13;17(1):225. doi: 10.1186/s13023-022-02368-9. Orphanet J Rare Dis. 2022. PMID: 35698232 Free PMC article.
References
-
- Ando H., Mitzutani A., Kiefer H., Tsuzurugi D., Michikawa T., Mikoshiba K. (2006). IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol. Cell 22, 795-806 - PubMed
-
- Berridge M. J. (2004). Conformational coupling: a physiological calcium entry mechanism. Sci. STKE 243, pe33 - PubMed
-
- Dellis O., Dedos S. G., Tovey S. C., Ur-Rahman T., Dubel S. J., Taylor C. W. (2006). Ca2+ entry through plasma membrane IP3 receptors. Science 313, 229-233 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

