Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex
- PMID: 21062901
- PMCID: PMC3016907
- DOI: 10.1093/hmg/ddq491
Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex
Abstract
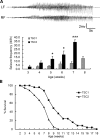
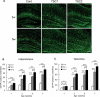
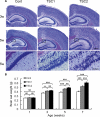
Tuberous Sclerosis Complex (TSC) is an autosomal dominant, multi-system disorder, typically involving severe neurological symptoms, such as epilepsy, cognitive deficits and autism. Two genes, TSC1 and TSC2, encoding the proteins hamartin and tuberin, respectively, have been identified as causing TSC. Although there is a substantial overlap in the clinical phenotype produced by TSC1 and TSC2 mutations, accumulating evidence indicates that TSC2 mutations cause more severe neurological manifestations than TSC1 mutations. In this study, the neurological phenotype of a novel mouse model involving conditional inactivation of the Tsc2 gene in glial-fibrillary acidic protein (GFAP)-positive cells (Tsc2(GFAP1)CKO mice) was characterized and compared with previously generated Tsc1(GFAP1)CKO mice. Similar to Tsc1(GFAP1)CKO mice, Tsc2(GFAP1)CKO mice exhibited epilepsy, premature death, progressive megencephaly, diffuse glial proliferation, dispersion of hippocampal pyramidal cells and decreased astrocyte glutamate transporter expression. However, Tsc2(GFAP1)CKO mice had an earlier onset and higher frequency of seizures, as well as significantly more severe histological abnormalities, compared with Tsc1(GFAP1)CKO mice. The differences between Tsc1(GFAP1)CKO and Tsc2(GFAP1)CKO mice were correlated with higher levels of mammalian target of rapamycin (mTOR) activation in Tsc2(GFAP1)CKO mice and were reversed by the mTOR inhibitor, rapamycin. These findings provide novel evidence in mouse models that Tsc2 mutations intrinsically cause a more severe neurological phenotype than Tsc1 mutations and suggest that the difference in phenotype may be related to the degree to which Tsc1 and Tsc2 inactivation causes abnormal mTOR activation.
Figures
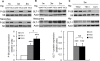
References
-
- Kwiatkowski D.J. Tuberous sclerosis: from tubers to mTOR. Ann. Hum. Genet. 2003;67:87–96. - PubMed
-
- Crino P.B., Nathanson K.L., Henske E.P. The tuberous sclerosis complex. N. Engl. J. Med. 2006;355:1345–1356. - PubMed
-
- Holmes G.L., Stafstrom C.E. and the Tuberous Sclerosis Study Group. Tuberous Sclerosis Complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48:617–630. - PubMed
-
- Jones A.C., Daniells C.E., Snell R.G., Tachataki M., Idziaszczyk S.A., Krawczak M., Sampson J.R., Cheadle J.P. Molecular genetic and phenotypic analysis reveals differences between TSC1 and TSC2 associated familial and sporadic tuberous sclerosis. Hum. Mol. Genet. 1997;6:2155–2161. - PubMed
-
- Dabora S.L., Jozwiak S., Franz D.N., Roberts P.S., Nieto A., Chung J., Coy Y.S., Reeve M.P., Thiele E., Egelhoff J.C., et al. Mutational analysis in a chort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am. J. Hum. Genet. 2001;68:64–80. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

