Expression and silencing of the microtubule-associated protein Tau in breast cancer cells
- PMID: 21062914
- PMCID: PMC3065197
- DOI: 10.1158/1535-7163.MCT-10-0780
Expression and silencing of the microtubule-associated protein Tau in breast cancer cells
Abstract
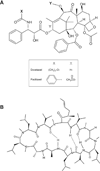
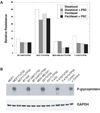
The microtubule-associated protein Tau has been reported to be a predictive factor for clinical response to taxanes in metastatic breast cancer. We generated a panel of eight taxane-resistant variants from four human breast cancer cell lines (MCF-7, T-47D, MDA-MB-231, and BT-549). Four variants had higher levels of Tau compared with their T-47D and MDA-MB-231 parental cells. Using isoform-specific primers, we found that Tau 0N, 1N, 2N, 3R, and 4R isoforms are overexpressed in the resistant variants, as is Tau exon 6 but not exons 4A or 8. To determine whether Tau overexpression produces resistance to taxanes, we derived three independent T-47D clones stably overexpressing Tau 3R and 4R isoforms. Tau overexpression did not result in taxane resistance compared with parental cells transfected with vector alone. We then knocked down Tau expression in three cell lines that expressed Tau constitutively (MCF-7 and ZR-75-1 breast cancer cells, and OVCAR-3 ovarian cancer cells). Lentivirus-mediated silencing of Tau expression in MCF-7 and OVCAR-3 cells did not result in increased taxane sensitivity compared with luciferase short hairpin RNA-infected cells and uninfected parental cells. Transient silencing using Tau-specific small interfering RNAs also did not alter taxane sensitivity relative to nontargeting controls in both MCF-7 and ZR-75-1 cells. These results show that neither overexpression nor depletion of Tau modulates cellular sensitivity to taxanes. Although Tau overexpression has been reported to be a predictive marker of taxane resistance, it is not likely to be a direct mechanism of taxane resistance in breast cancer.
©2010 AACR.
Figures


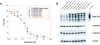

References
-
- Ring AE, Ellis PA. Taxanes in the treatment of early breast cancer. Cancer Treat Rev. 2005;31:618–627. - PubMed
-
- Bria E, Nistico C, Cuppone F, et al. Benefit of taxanes as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patients. Cancer. 2006;106:2337–2344. - PubMed
-
- Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

