A sense of urgency: Evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies
- PMID: 21062929
- PMCID: PMC3050630
- DOI: 10.1158/1078-0432.CCR-10-0133
A sense of urgency: Evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies
Abstract
Purpose: Postactivation barriers to oncology clinical trial accruals are well documented; however, potential barriers prior to trial opening are not. We investigate one such barrier: trial development time.
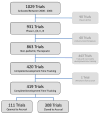
Experimental design: National Cancer Institute Cancer Therapy Evaluation Program (CTEP)-sponsored trials for all therapeutic, nonpediatric phase I, I/II, II, and III studies activated between 2000 and 2004 were investigated for an 8-year period (n = 419). Successful trials were those achieving 100% of minimum accrual goal. Time to open a study was the calendar time from initial CTEP submission to trial activation. Multivariate logistic regression analysis was used to calculate unadjusted and adjusted odds ratios (OR), controlling for study phase and size of expected accruals.
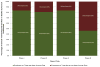
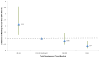
Results: Among the CTEP-approved oncology trials, 37.9% (n = 221) failed to attain the minimum accrual goals, with 70.8% (n = 14) of phase III trials resulting in poor accrual. A total of 16,474 patients (42.5% of accruals) accrued to those studies were unable to achieve the projected minimum accrual goal. Trials requiring less than 12 months of development were significantly more likely to achieve accrual goals (OR, 2.15; 95% confidence interval, 1.29-3.57, P = 0.003) than trials with the median development times of 12 to 18 months. Trials requiring a development time of greater than 24 months were significantly less likely to achieve accrual goals (OR, 0.40; 95% confidence interval, 0.20-0.78; P = 0.011) than trials with the median development time.
Conclusions: A large percentage of oncology clinical trials do not achieve minimum projected accruals. Trial development time appears to be one important predictor of the likelihood of successfully achieving the minimum accrual goals.
©2010 AACR.
Conflict of interest statement
The authors indicate no potential conflicts of interest
Figures
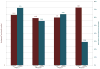
Comment in
-
Clinical trial development as a predictor of accrual performance--letter.Clin Cancer Res. 2011 Jul 1;17(13):4614; author reply 4615. doi: 10.1158/1078-0432.CCR-10-3418. Epub 2011 Jun 28. Clin Cancer Res. 2011. PMID: 21712458 No abstract available.
References
-
- SEER cancer statistics review. 2008.
-
- Heron M, Hoyert D, Xu J, Scott C, Tejada-Vera B. Deaths: Preliminary data for 2006. National vital statistics reports. 2008;56:1–52. - PubMed
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. Jama. 2004;291:2720–6. - PubMed
-
- Petrelli NJ, Grubbs S, Price K. Clinical trial investigator status: you need to earn it. J Clin Oncol. 2008;26:2440–1. - PubMed
-
- Go RS, Frisby KA, Lee JA, et al. Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer. 2006;106:426–33. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

