Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models
- PMID: 21062955
- PMCID: PMC3035679
- DOI: 10.1194/jlr.R009761
Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models
Abstract
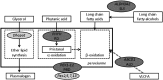
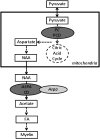
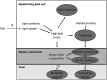
The integrity of central and peripheral nervous system myelin is affected in numerous lipid metabolism disorders. This vulnerability was so far mostly attributed to the extraordinarily high level of lipid synthesis that is required for the formation of myelin, and to the relative autonomy in lipid synthesis of myelinating glial cells because of blood barriers shielding the nervous system from circulating lipids. Recent insights from analysis of inherited lipid disorders, especially those with prevailing lipid depletion and from mouse models with glia-specific disruption of lipid metabolism, shed new light on this issue. The particular lipid composition of myelin, the transport of lipid-associated myelin proteins, and the necessity for timely assembly of the myelin sheath all contribute to the observed vulnerability of myelin to perturbed lipid metabolism. Furthermore, the uptake of external lipids may also play a role in the formation of myelin membranes. In addition to an improved understanding of basic myelin biology, these data provide a foundation for future therapeutic interventions aiming at preserving glial cell integrity in metabolic disorders.
Figures
References
-
- Barres B. A. 2008. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 60: 430–440. - PubMed
-
- Waxman S. G. 1980. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 3: 141–150. - PubMed
-
- Hartline D. K., Colman D. R. 2007. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr. Biol. 17: R29–R35. - PubMed
-
- Norton W. T., Poduslo S. E. 1973. Myelination in rat brain: method of myelin isolation. J. Neurochem. 21: 749–757. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

