Induction of human epithelial stem/progenitor expansion by FOXM1
- PMID: 21062979
- PMCID: PMC3044465
- DOI: 10.1158/0008-5472.CAN-10-2173
Induction of human epithelial stem/progenitor expansion by FOXM1
Erratum in
- Cancer Res. 2011 Jan 1;71(1):290
Abstract
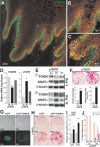
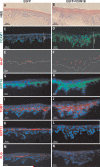
Stem cells are permanent residents of tissues and thought to be targets of cancer initiation. The frequent, and often early, upregulation of the FOXM1 transcription factor in the majority of human cancers suggests that it may participate in the initiation of human tumorigenesis. However, this hypothesis has not been tested. Herein, we show that targeting the ectopic expression of FOXM1 to the highly clonogenic cells of primary human keratinocytes with stem/progenitor cell properties, but not to differentiating cells, caused clonal expansion in vitro. We show, using a functional three-dimensional organotypic epithelial tissue regeneration system, that ectopic FOXM1 expression perturbed epithelial differentiation generating a hyperproliferative phenotype reminiscent of that seen in human epithelial hyperplasia. Furthermore, transcriptional expression analysis of a panel of 28 epithelial differentiation-specific genes reveals a role for FOXM1 in the suppression of epithelial differentiation. This study provides the first evidence that FOXM1 participates in an early oncogenic pathway that predisposes cells to tumorigenesis by expanding the stem/progenitor compartment and deregulating subsequent keratinocyte terminal differentiation. This finding reveals an important window of susceptibility to oncogenic signals in epithelial stem/progenitor cells prior to differentiation, and may provide a significant benefit to the design of cancer therapeutic interventions that target oncogenesis at its earliest incipient stage.
Copyright © 2010 AACR.
Figures



References
-
- Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7(11):847–59. - PubMed
-
- Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388(12):1257–74. - PubMed
-
- Laoukili J, Kooistra MR, Bras A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7(2):126–36. - PubMed
-
- Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res. 2002;62(16):4773–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

