Modulation of ghrelin O-acyltransferase expression in pancreatic islets
- PMID: 21063108
- PMCID: PMC3048940
- DOI: 10.1159/000322338
Modulation of ghrelin O-acyltransferase expression in pancreatic islets
Abstract
Background: Ghrelin, the only identified circulating orexigenic signal, is unique in structure in which a specific acyl-modification of its third serine occurs. This acylation is necessary for ghrelin to bind to its receptor and to exert its biologic activity, which is catalyzed by ghrelin O-acyltransferase (GOAT). Although ghrelin is mainly secreted from gastric X/A like endocrine cells, it is also expressed in pancreatic islet cells and regulates insulin secretion. In this study, we examined the expression and regulation of GOAT in pancreas.
Methods: GOAT mRNA and immunoreactivity were examined in pancreatic islets and INS-1 cells by RT-PCR and immunofluorescent staining or Western blotting.
Results: Insulin inhibits the expression of GOAT mRNA and GOAT promoter activity in a dose and time-dependent manner. The mammalian target of rapamycin (mTOR) is activated by insulin. Blocking mTOR signaling by either rapamycin or overexpression of its negative regulator tuberous sclerosis complex 1 (TSC1) or TSC2 attenuates the inhibitory effect of insulin on the transcription and translation of GOAT.
Conclusion: Our study suggests that GOAT is present in pancreatic islet cells and that insulin inhibits the expression of GOAT via the mediation of mTOR signaling.
Copyright © 2010 S. Karger AG, Basel.
Figures





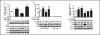
Similar articles
-
Ghrelin, ghrelin-O-acyl transferase, nucleobindin-2/nesfatin-1 and prohormone convertases in the pancreatic islets of Sprague Dawley rats during development.J Mol Histol. 2016 Jun;47(3):325-36. doi: 10.1007/s10735-016-9673-4. Epub 2016 Apr 12. J Mol Histol. 2016. PMID: 27071923
-
ETV5 regulates GOAT/ghrelin system in an mTORC1-dependent manner.Mol Cell Endocrinol. 2019 Apr 5;485:72-80. doi: 10.1016/j.mce.2019.02.003. Epub 2019 Feb 5. Mol Cell Endocrinol. 2019. PMID: 30735697
-
Discovery of ghrelin o-acyltransferase.Endocr Dev. 2013;25:16-24. doi: 10.1159/000346039. Epub 2013 Apr 25. Endocr Dev. 2013. PMID: 23652388
-
The ghrelin O-acyltransferase-ghrelin system: a novel regulator of glucose metabolism.Curr Opin Endocrinol Diabetes Obes. 2011 Feb;18(1):50-5. doi: 10.1097/MED.0b013e328341e1d3. Curr Opin Endocrinol Diabetes Obes. 2011. PMID: 21150588 Review.
-
Ghrelin O-acyltransferase (GOAT) and energy metabolism.Sci China Life Sci. 2016 Mar;59(3):281-91. doi: 10.1007/s11427-015-4973-6. Epub 2016 Jan 6. Sci China Life Sci. 2016. PMID: 26732975 Review.
Cited by
-
Ghrelin O Acyl Transferase (GOAT) as a Novel Metabolic Regulatory Enzyme.J Clin Diagn Res. 2015 Feb;9(2):LE01-5. doi: 10.7860/JCDR/2015/9787.5514. Epub 2015 Feb 1. J Clin Diagn Res. 2015. PMID: 25859472 Free PMC article. Review.
-
Ghrelin contributes to derangements of glucose metabolism induced by rapamycin in mice.Diabetologia. 2012 Jun;55(6):1813-23. doi: 10.1007/s00125-012-2509-1. Epub 2012 Mar 3. Diabetologia. 2012. PMID: 22391948 Free PMC article.
-
The ghrelin O-acyltransferase-ghrelin system reduces TNF-α-induced apoptosis and autophagy in human visceral adipocytes.Diabetologia. 2012 Nov;55(11):3038-50. doi: 10.1007/s00125-012-2671-5. Epub 2012 Aug 7. Diabetologia. 2012. PMID: 22869322
-
Ghrelin, ghrelin-O-acyl transferase, nucleobindin-2/nesfatin-1 and prohormone convertases in the pancreatic islets of Sprague Dawley rats during development.J Mol Histol. 2016 Jun;47(3):325-36. doi: 10.1007/s10735-016-9673-4. Epub 2016 Apr 12. J Mol Histol. 2016. PMID: 27071923
-
Physiologic concentrations of exogenously infused ghrelin reduces insulin secretion without affecting insulin sensitivity in healthy humans.J Clin Endocrinol Metab. 2013 Jun;98(6):2536-43. doi: 10.1210/jc.2012-4162. Epub 2013 Apr 15. J Clin Endocrinol Metab. 2013. PMID: 23589527 Free PMC article.
References
-
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. - PubMed
-
- Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85:495–522. - PubMed
-
- Zhang W, Hu Y, Lin TR, Fan Y, Mulholland MW. Stimulation of neurogenesis in rat nucleus of the solitary tract by ghrelin. Peptides. 2005;26:2280–2288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
