Acid sphingomyelinase deficiency attenuates bleomycin-induced lung inflammation and fibrosis in mice
- PMID: 21063112
- PMCID: PMC3048941
- DOI: 10.1159/000322342
Acid sphingomyelinase deficiency attenuates bleomycin-induced lung inflammation and fibrosis in mice
Abstract
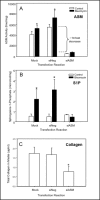
Background/aims: The sphingomyelin/ceramide signaling pathway is an important component of many cellular processes implicated in the pathogenesis of lung disease. Acid sphingomyelinase (ASM) is a key mediator of this pathway, but its specific role in pulmonary fibrosis has not been previously investigated. Here we used the bleomycin model of pulmonary fibrosis to investigate fibrotic responses in normal and ASM knockout (ASM(-/-)) mice, and in NIH3T3 fibroblasts with and without ASM siRNA treatment.
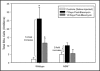
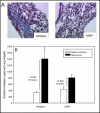
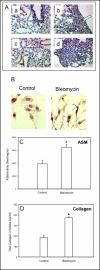
Methods: Mice and cells with and without ASM activity were treated with bleomycin, and the effects on lung inflammation, formation of collagen producing myofibroblasts, and apoptosis were assessed.
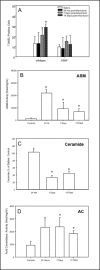
Results: The development of bleomycin-induced inflammation and fibrosis in wildtype mice correlated with the rapid activation of ASM, and was markedly attenuated in the absence of ASM activity. Along with the elevated ASM activity, there also was an elevation of acid ceramidase (AC) activity, which was sustained for up to 14 days post-bleomycin treatment. Studies in NIH3T3 fibroblasts confirmed these findings, and revealed a direct effect of ASM/AC activation on the formation of myofibroblasts. Cell studies also showed that a downstream effect of bleomycin treatment was the production of sphingosine-1-phosphate.
Conclusions: These data demonstrate that the sphingomyelin/ceramide signaling pathway is involved in the pathogenesis of bleomycin-induced pulmonary fibrosis, and suggest that inhibition of ASM may potentially slow the fibrotic process in the lung.
Copyright © 2010 S. Karger AG, Basel.
Figures

References
-
- Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J. 2007;30:835–839. - PubMed
-
- Hauber HP, Blaukovitsch M. Current and future treatment options in idiopathic pulmonary fibrosis. Inflamm Allergy Drug Targets. 2010 in press. - PubMed
-
- Chua F, Gauldie J, Laurent GL. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33:9–13. - PubMed
-
- Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao A, Polosukhin V, Wain J, Karimi-Shah B, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. - PubMed
-
- Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
