Heparin use in a rat hemorrhagic shock model induces biologic activity in mesenteric lymph separate from shock
- PMID: 21063238
- PMCID: PMC3089771
- DOI: 10.1097/SHK.0b013e31820239ee
Heparin use in a rat hemorrhagic shock model induces biologic activity in mesenteric lymph separate from shock
Abstract
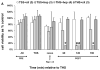
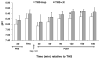
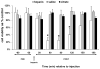
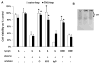
Experimental data have shown that mesenteric lymph from rats subjected to trauma-hemorrhagic shock (THS) but not trauma-sham shock induces neutrophil activation, cytotoxicity, decreased red blood cell (RBC) deformability, and bone marrow colony growth suppression. These data have led to the hypothesis that gut factors produced from THS enter the systemic circulation via the mesenteric lymphatics and contribute to the progression of multiple organ failure after THS. Ongoing studies designed to identify bioactive lymph agents implicated factors associated with the heparin use in the THS procedure. We investigated if heparin itself was responsible for reported toxicity to human umbilical vein endothelial cells (HUVECs). Human umbilical vein endothelial cell toxicity was not induced by lymph when alternate anticoagulants (citrate and EDTA) were used in THS. Human umbilical vein endothelial cell toxicity was induced by lymph after heparin but not saline or citrate injection into trauma-sham shock and naive animals and was dose dependent. Activities of both heparin-releasable lipases (lipoprotein and hepatic) were detected in the plasma and lymph from THS and naive animals receiving heparin but not citrate or saline. Lymph-induced HUVEC toxicity correlated with lymph lipase activities. Finally, incubation of HUVECs with purified lipoprotein lipase added to naive lymph-induced toxicity in vitro. These data show that heparin, not THS, is responsible for the reported lymph-mediated HUVEC toxicity through its release of lipases into the lymph. These findings can provide alternative explanations for several of the THS effects reported in the literature using heparin models, thus necessitating a review of previous work in this field.
Conflict of interest statement
Figures













Similar articles
-
Anticoagulants influence the in vitro activity and composition of shock lymph but not its in vivo activity.Shock. 2011 Aug;36(2):177-83. doi: 10.1097/SHK.0b013e3182205c30. Shock. 2011. PMID: 21558984 Free PMC article.
-
Trauma-hemorrhagic shock mesenteric lymph from rat contains a modified form of albumin that is implicated in endothelial cell toxicity.Shock. 2005 May;23(5):417-25. doi: 10.1097/01.shk.0000160524.14235.6c. Shock. 2005. PMID: 15834307
-
Trauma-hemorrhagic shock mesenteric lymph induces endothelial apoptosis that involves both caspase-dependent and caspase-independent mechanisms.Ann Surg. 2004 Jul;240(1):123-31. doi: 10.1097/01.sla.0000129341.94219.cf. Ann Surg. 2004. PMID: 15213628 Free PMC article.
-
Role of the gut lymphatic system in multiple organ failure.Curr Opin Crit Care. 2001 Apr;7(2):92-8. doi: 10.1097/00075198-200104000-00007. Curr Opin Crit Care. 2001. PMID: 11373517 Review.
-
[Induction mechanism of shock: applying the etiology in judgment of the cause of death in forensic practice].Nihon Hoigaku Zasshi. 2004 Sep;58(2):130-40. Nihon Hoigaku Zasshi. 2004. PMID: 15526767 Review. Japanese.
Cited by
-
Redefining the gut as the motor of critical illness.Trends Mol Med. 2014 Apr;20(4):214-23. doi: 10.1016/j.molmed.2013.08.004. Epub 2013 Sep 18. Trends Mol Med. 2014. PMID: 24055446 Free PMC article. Review.
-
Role of lipase-generated free fatty acids in converting mesenteric lymph from a noncytotoxic to a cytotoxic fluid.Am J Physiol Gastrointest Liver Physiol. 2012 Oct 15;303(8):G969-78. doi: 10.1152/ajpgi.00290.2012. Epub 2012 Aug 16. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22899820 Free PMC article.
-
Anticoagulants influence the in vitro activity and composition of shock lymph but not its in vivo activity.Shock. 2011 Aug;36(2):177-83. doi: 10.1097/SHK.0b013e3182205c30. Shock. 2011. PMID: 21558984 Free PMC article.
-
Why Do Men Accumulate Abdominal Visceral Fat?Front Physiol. 2019 Dec 5;10:1486. doi: 10.3389/fphys.2019.01486. eCollection 2019. Front Physiol. 2019. PMID: 31866877 Free PMC article.
-
Activated platelets in heparinized shed blood: the "second hit" of acute lung injury in trauma/hemorrhagic shock models.Shock. 2011 Dec;36(6):595-603. doi: 10.1097/SHK.0b013e318231ee76. Shock. 2011. PMID: 21841533 Free PMC article.
References
-
- Deitch EA, Goodman E. Prevention of Multiple Organ Failure. Surg Clin North Am. 1999;79(6):1471–1488. - PubMed
-
- Glenn TM, Lefer AM. Protective effect of thoracic lymph diversion in hemorrhagic shock. Am J Physiol. 1970;219(5):1305–1310. - PubMed
-
- Papp M, Nemeth E, Feuer I, Fodor I. Effect of an impairment of lymph flow on experimental acute “pancreatitis”. Acta Med Acad Sci Hung. 1957;11(2):203–208. - PubMed
-
- Deitch EA, Xu DZ, Kaiser VL. Role of the gut in development of injury and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

