A physiological model for autonomic heart rate regulation in human endotoxemia
- PMID: 21063241
- PMCID: PMC3045969
- DOI: 10.1097/SHK.0b013e318200032b
A physiological model for autonomic heart rate regulation in human endotoxemia
Abstract
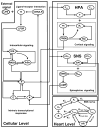
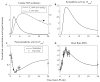
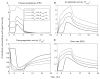
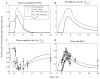
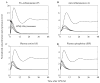
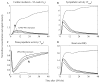
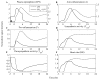
The systemic inflammatory response syndrome often accompanies critical illnesses and can be an important cause of morbidity and mortality. Marked abnormalities in cardiovascular function accompany acute illnesses manifested as sustained tachyarrhythmias, which are but one component of systemic dysregulation. The realization that cardiac pacemaker activity is under control of the autonomic nervous system has promoted the analysis of heart rate (HR) variation for assessing autonomic activities. In acute illnesses, autonomic imbalance manifesting in part as parasympathetic attenuation is associated with increased morbidity in patients who manifest systemic inflammatory response syndrome phenotype. Driven by the premise that biological phenotypes emerge as the outcome of the coordinated action of network elements across the host, a multiscale model of human endotoxemia, as a prototype model of systemic inflammation in humans, is developed that quantifies critical aspects of the complex relationship between inflammation and autonomic HR regulation. In the present study, changes in HR response to acute injury, phenotypically expressed as tachycardia, are simulated as a result of autonomic imbalance that reflects sympathetic activity excess and parasympathetic attenuation. The proposed model assesses both the anti-inflammatory and cardiovascular effects of antecedent stresses upon the systemic inflammatory manifestations of human endotoxemia as well as a series of nonlinear inflammatory relevant scenarios. Such a modeling approach provides a comprehensive conceptual framework linking inflammation and physiological complexity via a multiscale model that may advance the translational potential of systems modeling in clinical research.
Figures
Similar articles
-
Translational applications of evaluating physiologic variability in human endotoxemia.J Clin Monit Comput. 2013 Aug;27(4):405-15. doi: 10.1007/s10877-012-9418-1. Epub 2012 Dec 1. J Clin Monit Comput. 2013. PMID: 23203205 Free PMC article. Review.
-
Multiscale model for the assessment of autonomic dysfunction in human endotoxemia.Physiol Genomics. 2010 Jun;42(1):5-19. doi: 10.1152/physiolgenomics.00184.2009. Epub 2010 Mar 16. Physiol Genomics. 2010. PMID: 20233835 Free PMC article.
-
On heart rate variability and autonomic activity in homeostasis and in systemic inflammation.Math Biosci. 2014 Jun;252:36-44. doi: 10.1016/j.mbs.2014.03.010. Epub 2014 Mar 26. Math Biosci. 2014. PMID: 24680646 Free PMC article.
-
Modeling autonomic regulation of cardiac function and heart rate variability in human endotoxemia.Physiol Genomics. 2011 Aug 24;43(16):951-64. doi: 10.1152/physiolgenomics.00040.2011. Epub 2011 Jun 14. Physiol Genomics. 2011. PMID: 21673075 Free PMC article.
-
Heart rate variability in critical care medicine.Curr Opin Crit Care. 2002 Oct;8(5):371-5. doi: 10.1097/00075198-200210000-00002. Curr Opin Crit Care. 2002. PMID: 12357103 Review.
Cited by
-
In silico modeling: methods and applications to trauma and sepsis.Crit Care Med. 2013 Aug;41(8):2008-14. doi: 10.1097/CCM.0b013e31829a6eb4. Crit Care Med. 2013. PMID: 23863232 Free PMC article. Review.
-
Mathematical modeling of temperature-induced circadian rhythms.Front Syst Biol. 2024 Mar 25;4:1256398. doi: 10.3389/fsysb.2024.1256398. eCollection 2024. Front Syst Biol. 2024. PMID: 40809122 Free PMC article.
-
Translational applications of evaluating physiologic variability in human endotoxemia.J Clin Monit Comput. 2013 Aug;27(4):405-15. doi: 10.1007/s10877-012-9418-1. Epub 2012 Dec 1. J Clin Monit Comput. 2013. PMID: 23203205 Free PMC article. Review.
-
Computational modelling of the inflammatory response in trauma, sepsis and wound healing: implications for modelling resilience.Interface Focus. 2014 Oct 6;4(5):20140004. doi: 10.1098/rsfs.2014.0004. Interface Focus. 2014. PMID: 25285195 Free PMC article. Review.
-
Light-induced synchronization of the SCN coupled oscillators and implications for entraining the HPA axis.Front Endocrinol (Lausanne). 2022 Oct 27;13:960351. doi: 10.3389/fendo.2022.960351. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36387856 Free PMC article.
References
-
- Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006;12:151–70. - PubMed
-
- Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–7. - PubMed
-
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

