Cost-effective and compact wide-field fluorescent imaging on a cell-phone
- PMID: 21063582
- PMCID: PMC3073081
- DOI: 10.1039/c0lc00358a
Cost-effective and compact wide-field fluorescent imaging on a cell-phone
Erratum in
- Lab Chip. 2011 Dec 21;11(24):4279
Abstract
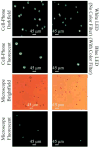
We demonstrate wide-field fluorescent and darkfield imaging on a cell-phone with compact, light-weight and cost-effective optical components that are mechanically attached to the existing camera unit of the cell-phone. For this purpose, we used battery powered light-emitting diodes (LEDs) to pump the sample of interest from the side using butt-coupling, where the pump light was guided within the sample cuvette to uniformly excite the specimen. The fluorescent emission from the sample was then imaged using an additional lens that was positioned right in front of the existing lens of the cell-phone camera. Because the excitation occurs through guided waves that propagate perpendicular to our detection path, an inexpensive plastic colour filter was sufficient to create the dark-field background required for fluorescent imaging, without the need for a thin-film interference filter. We validate the performance of this platform by imaging various fluorescent micro-objects in 2 colours (i.e., red and green) over a large field-of-view (FOV) of ∼81 mm(2) with a raw spatial resolution of ∼20 μm. With additional digital processing of the captured cell-phone images, through the use of compressive sampling theory, we demonstrate ∼2 fold improvement in our resolving power, achieving ∼10 μm resolution without a trade-off in our FOV. Further, we also demonstrate darkfield imaging of non-fluorescent specimen using the same interface, where this time the scattered light from the objects is detected without the use of any filters. The capability of imaging a wide FOV would be exceedingly important to probe large sample volumes (e.g., >0.1 mL) of e.g., blood, urine, sputum or water, and for this end we also demonstrate fluorescent imaging of labeled white-blood cells from whole blood samples, as well as water-borne pathogenic protozoan parasites such as Giardia Lamblia cysts. Weighing only ∼28 g (∼1 ounce), this compact and cost-effective fluorescent imaging platform attached to a cell-phone could be quite useful especially for resource-limited settings, and might provide an important tool for wide-field imaging and quantification of various lab-on-a-chip assays developed for global health applications, such as monitoring of HIV+ patients for CD4 counts or viral load measurements.
Figures






References
-
- International Telecommunication Union. Market information and statistics. 2010. http://www.itu.int/ITU-D/ict/statistics/index.html.
-
- Woodward B, Istepanian RSH, Richards CI. IEEE Trans Inf Technol Biomed. 2001;5:13–15. - PubMed
-
- Ruano-López JM, Agirregabiria M, Olabarria G, Verdoy D, Bang DD, Bu M, Wolff A, Voigt A, Dziuban JA, Walczak R, Berganzo J. Lab Chip. 2009;9:1495–1499. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

