Functional impact of PTP1B-mediated Src regulation on oxidative phosphorylation in rat brain mitochondria
- PMID: 21063895
- PMCID: PMC11115002
- DOI: 10.1007/s00018-010-0573-6
Functional impact of PTP1B-mediated Src regulation on oxidative phosphorylation in rat brain mitochondria
Abstract
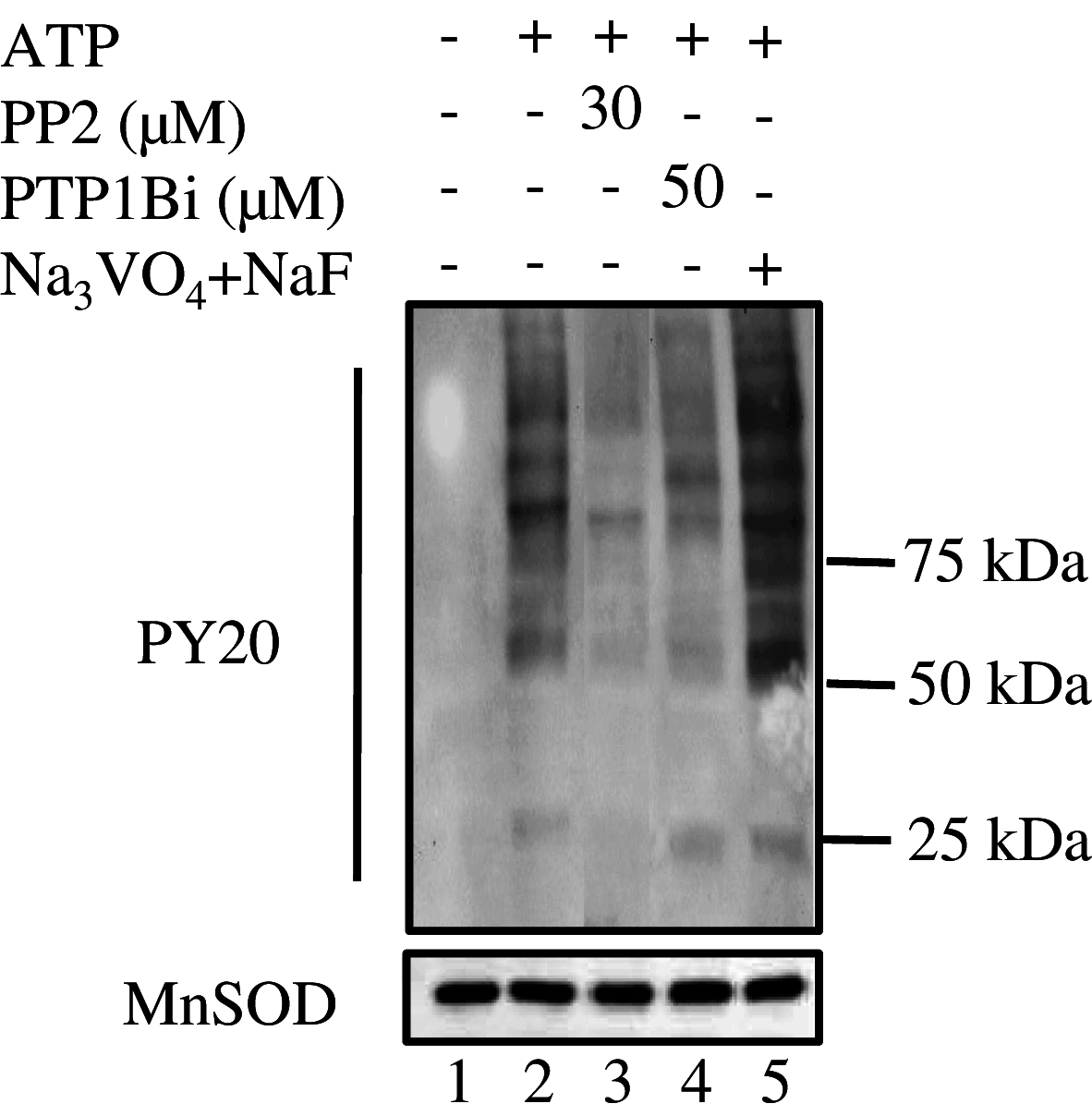
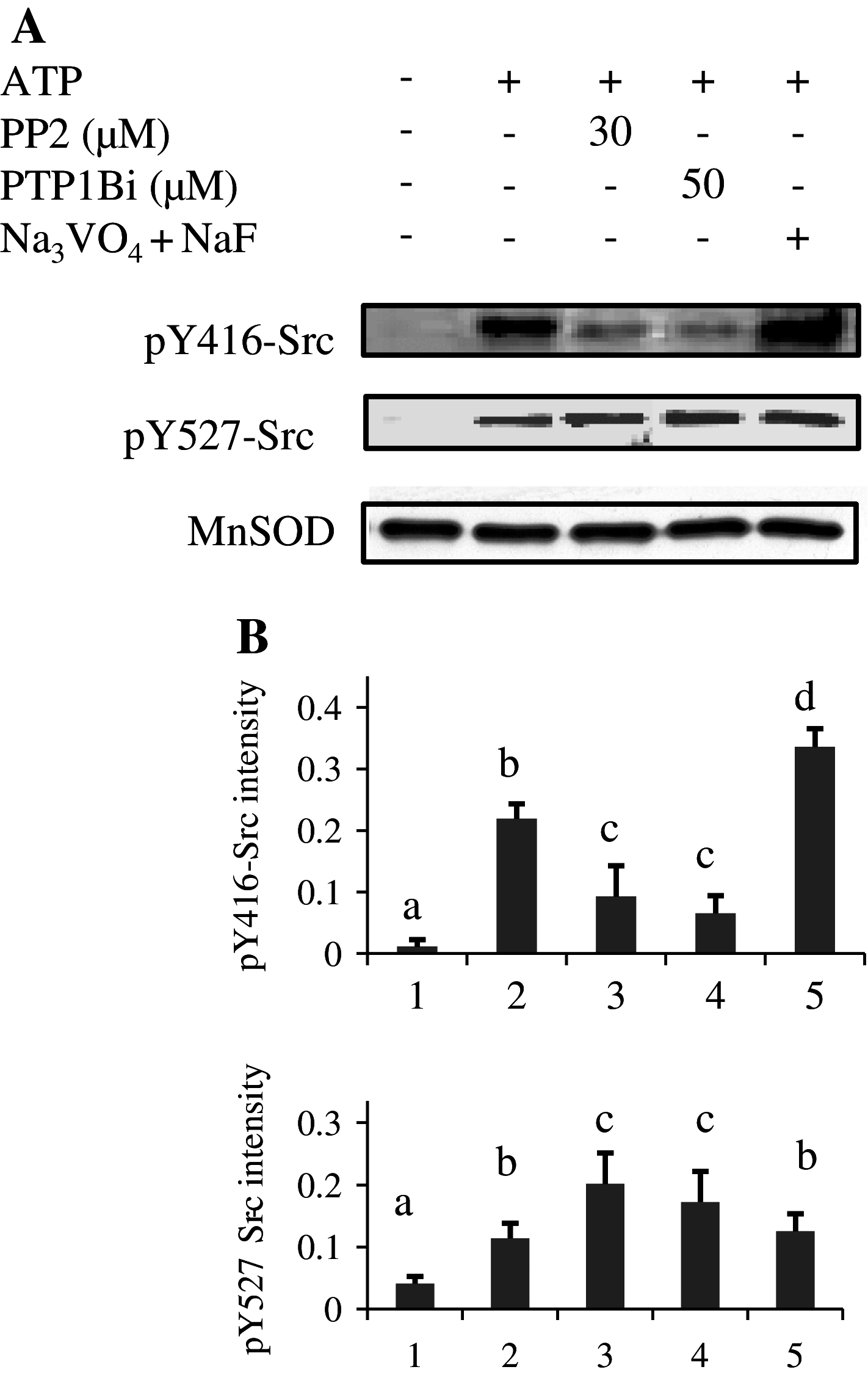
Given the presence of Src and PTP1B within rat brain mitochondria, we have investigated whether PTP1B regulates Src activity in mitochondria as in the cytosol. Results showed that Src was stimulated by in vitro addition of ATP to mitochondria, and this stimulation was reversed by a membrane-permeable allosteric inhibitor of PTP1B and by a potent selective Src inhibitor. They also indicated a direct action of PTP1B on phosphorylated tyrosine 527 residue of Src, thus implicating a role for PTP1B in the modulation of Src activity in mitochondria. Putative Src and PTP1B substrates were identified by liquid chromatography tandem mass spectrometry and two-dimensional blue native/SDS-PAGE. Both inhibitors inhibited ADP-stimulated respirations concurrently with Src activation and complex IV activation by ATP, while having no effect or increasing the activity of the other complexes. Our analysis emphasizes the regulatory function of Src and its modulation by PTP1B on oxidative phosphorylation in mitochondria.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

