Prebiotic synthesis of methionine and other sulfur-containing organic compounds on the primitive Earth: a contemporary reassessment based on an unpublished 1958 Stanley Miller experiment
- PMID: 21063908
- PMCID: PMC3094541
- DOI: 10.1007/s11084-010-9228-8
Prebiotic synthesis of methionine and other sulfur-containing organic compounds on the primitive Earth: a contemporary reassessment based on an unpublished 1958 Stanley Miller experiment
Abstract
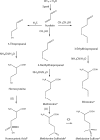
Original extracts from an unpublished 1958 experiment conducted by the late Stanley L. Miller were recently found and analyzed using modern state-of-the-art analytical methods. The extracts were produced by the action of an electric discharge on a mixture of methane (CH(4)), hydrogen sulfide (H(2)S), ammonia (NH(3)), and carbon dioxide (CO(2)). Racemic methionine was formed in significant yields, together with other sulfur-bearing organic compounds. The formation of methionine and other compounds from a model prebiotic atmosphere that contained H(2)S suggests that this type of synthesis is robust under reducing conditions, which may have existed either in the global primitive atmosphere or in localized volcanic environments on the early Earth. The presence of a wide array of sulfur-containing organic compounds produced by the decomposition of methionine and cysteine indicates that in addition to abiotic synthetic processes, degradation of organic compounds on the primordial Earth could have been important in diversifying the inventory of molecules of biochemical significance not readily formed from other abiotic reactions, or derived from extraterrestrial delivery.
Figures



References
-
- Bada JL. Amino acid cosmogeochemistry. Philos Trans: Biol Sci. 1991;333:349–358. doi: 10.1098/rstb.1991.0084. - DOI
-
- Choughuley ASU, Lemmon RM. Production of cysteic acid, taurine and cystamine under primitive earth conditions. Nature. 1966;210:628–629. doi: 10.1038/210628a0. - DOI
-
- Cleaves HJ. The prebiotic synthesis of acrolein. Monatsh Chem. 2003;134:585–593.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

