Key signaling nodes in mammary gland development and cancer: β-catenin
- PMID: 21067528
- PMCID: PMC3046427
- DOI: 10.1186/bcr2723
Key signaling nodes in mammary gland development and cancer: β-catenin
Abstract
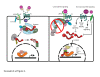
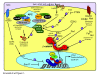
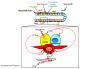
β-Catenin plays important roles in mammary development and tumorigenesis through its functions in cell adhesion, signal transduction and regulation of cell-context-specific gene expression. Studies in mice have highlighted the critical role of β-catenin signaling for stem cell biology at multiple stages of mammary development. Deregulated β-catenin signaling disturbs stem and progenitor cell dynamics and induces mammary tumors in mice. Recent data showing deregulated β-catenin signaling in metaplastic and basal-type tumors suggest a similar link to reactivated developmental pathways and human breast cancer. The present review will discuss β-catenin as a central transducer of numerous signaling pathways and its role in mammary development and breast cancer.
Figures
Similar articles
-
SOX9 regulates low density lipoprotein receptor-related protein 6 (LRP6) and T-cell factor 4 (TCF4) expression and Wnt/β-catenin activation in breast cancer.J Biol Chem. 2013 Mar 1;288(9):6478-87. doi: 10.1074/jbc.M112.419184. Epub 2013 Jan 10. J Biol Chem. 2013. PMID: 23306204 Free PMC article.
-
Wnt signaling, stem cells, and the cellular origin of breast cancer.Stem Cell Rev. 2007 Jun;3(2):157-68. doi: 10.1007/s12015-007-0025-3. Stem Cell Rev. 2007. PMID: 17873348 Review.
-
Human chorionic gonadotropin (hCG) up-regulates wnt5b and wnt7b in the mammary gland, and hCGbeta transgenic female mice present with mammary Gland tumors exhibiting characteristics of the Wnt/beta-catenin pathway activation.Endocrinology. 2007 Aug;148(8):3694-703. doi: 10.1210/en.2007-0249. Epub 2007 May 17. Endocrinology. 2007. PMID: 17510243
-
MiR-31 promotes mammary stem cell expansion and breast tumorigenesis by suppressing Wnt signaling antagonists.Nat Commun. 2017 Oct 19;8(1):1036. doi: 10.1038/s41467-017-01059-5. Nat Commun. 2017. PMID: 29051494 Free PMC article.
-
Wnt and mammary stem cells: hormones cannot fly wingless.Curr Opin Pharmacol. 2010 Dec;10(6):643-9. doi: 10.1016/j.coph.2010.07.004. Curr Opin Pharmacol. 2010. PMID: 20810315 Free PMC article. Review.
Cited by
-
Stable inhibition of specific estrogen receptor α (ERα) phosphorylation confers increased growth, migration/invasion, and disruption of estradiol signaling in MCF-7 breast cancer cells.Endocrinology. 2012 Sep;153(9):4144-59. doi: 10.1210/en.2011-2001. Epub 2012 Jun 25. Endocrinology. 2012. PMID: 22733972 Free PMC article.
-
The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer.BMC Cancer. 2013 Apr 2;13:174. doi: 10.1186/1471-2407-13-174. BMC Cancer. 2013. PMID: 23547709 Free PMC article.
-
Pak1 kinase links ErbB2 to β-catenin in transformation of breast epithelial cells.Cancer Res. 2013 Jun 15;73(12):3671-82. doi: 10.1158/0008-5472.CAN-12-4453. Epub 2013 Apr 10. Cancer Res. 2013. PMID: 23576562 Free PMC article.
-
Whole genome DNA methylation signature of HER2-positive breast cancer.Epigenetics. 2014 Aug;9(8):1149-62. doi: 10.4161/epi.29632. Epub 2014 Jul 8. Epigenetics. 2014. PMID: 25089541 Free PMC article.
-
β-Catenin haploinsufficiency promotes mammary tumorigenesis in an ErbB2-positive basal breast cancer model.Proc Natl Acad Sci U S A. 2017 Jan 31;114(5):E707-E716. doi: 10.1073/pnas.1610383114. Epub 2017 Jan 17. Proc Natl Acad Sci U S A. 2017. PMID: 28096336 Free PMC article.
References
-
- Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, Peterse JL, Cardiff RD, Berns A, Jonkers J. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. - DOI - PubMed
-
- Nagi C, Guttman M, Jaffer S, Qiao R, Keren R, Triana A, Li M, Godbold J, Bleiweiss IJ, Hazan RB. N-cadherin expression in breast cancer: correlation with an aggressive histologic variant - invasive micropapillary carcinoma. Breast Cancer Res Treat. 2005;94:225–235. doi: 10.1007/s10549-005-7727-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

